Table Of Content36 Volume 36:
Alcohols, Alcoholates (ROM, M = metal)
Clayden, J., in Science of Synthesis, 36 (2007), p.1
Information on Science of Synthesis Volume 36
Feedback
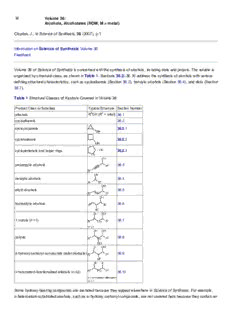
Volume 36 of Science of Synthesis is concerned with the synthesis of alcohols, including diols and polyols. The volume is
organized by structural class, as shown in Table 1. Sections 36.2–36.10 address the synthesis of alcohols with various
defining structural characteristics, such as cycloalkanols (Section 36.2), benzylic alcohols (Section 36.4), and diols (Section
36.7).
Table 1 Structural Classes of Alcohols Covered in Volume 36
Product Class or Subclass Typical Structure Section Number
alkanols R1OH (R1 = alkyl) 36.1
cycloalkanols 36.2
cyclopropanols 36.2.1
cyclobutanols 36.2.2
cyclopentanols and larger rings 36.2.3
propargylic alcohols 36.3
benzylic alcohols 36.4
allylic alcohols 36.5
homoallylic alcohols 36.6
1,n-diols (n >1) 36.7
polyols 36.8
β-hydroxy carbonyl compounds (aldol products) 36.9
n-heteroatom-functionalized alkanols (n ≥2) 36.10
Some hydroxy-bearing compounds are excluded because they appear elsewhere in Science of Synthesis. For example,
α-heteroatom-substituted alcohols, such as α-hydroxy carbonyl compounds, are not covered here because they contain an
additional functional group of higher priority than the hydroxy group under the Science of Synthesis classification system.
Coverage of these compounds can be found in the revelant volume that deals with the higher-priority group in question.
Section 36.1 is more general, dealing with the synthesis of alcohols of any structural class (including simple alkanols) and
is organized according to synthetic method, as shown in Table 2. Thus, a method might appear in Section 36.1 if it is
suitable for the synthesis of alcohols in general, but methods described in Sections 36.2–36.10 will be applicable primarily
to a more restricted class of alcohols. In a similar vein, Section 36.11 addresses the deprotection of protected alcohols.
Table 2 Methods for the Synthesis of Alkanols Covered in Volume 36
Section
Method Typical Reaction
Number
oxidation (metal and enzyme catalyzed) 36.1.1
reduction 36.1.2
substitution 36.1.3
addition to alkynes and alkenes 36.1.4
carbonylation reactions 36.1.5
addition of organometallics to carbon dioxide, carboxylic acids, and
36.1.6
derivatives
addition of organometallics to aldehydes and ketones 36.1.7
resolution or inversion 36.1.8
synthesis from other alcohols 36.1.9
deprotection 36.11
Finally, Section 36.12 covers metal alcoholates: compounds related to alcohols by replacement of the hydroxy hydrogen
by a metal.
The most conceptually simple synthesis of an alcohol imaginable would start with an alkane and introduce a hydroxy group.
This is a simple idea, the realization of which is an ongoing challenge to chemists, and this is where the chemistry of this
volume begins, with the use of oxygen in the presence of transition-metal catalysts to oxidize simple alkanes to alcohols. A
1 mol% quantity of iron catalyzes the conversion of adamantane (1) into adamantan-1-ol (2) using just oxygen in the
presence of an aldehyde (Scheme 1).[1]
Scheme 1 Alcohol Formation by Metal-Catalyzed Hydroxylation[1]
Oxidation of unactivated C—H bonds can be achieved in biological systems, giving biocatalytic methods the edge when it
comes to the regio- and chemoselective introduction of hydroxy groups in this way. Biological methods may be limited in
generality, but protective modification of the substrate to suit a specific organism can be used to promote otherwise
challenging oxidations. For example, cyclopentanecarboxylic acid can be oxidized regio- and stereospecifically to yield
alcohol 4 when protected as its benzoxazole derivative 3 (Scheme 2).[2–5]
Scheme 2 Biocatalytic Hydroxylation Using Biocompatible Protection[2–5]
Selective oxidation methods allow the use of C—Si, C—B, and carbon—metal bonds as precursors to C—O bonds, and
Section 36.1.1 covers the important synthetic strategy of using silanes, and in particular phenyldimethylsilanes, as masked
alcohols. Thus, alcohol 6 is formed stereospecifically from silane 5 (Scheme 3).[6] Directed metalation chemistry permits
the regioselective introduction of metals, which may then be transformed into hydroxy groups simply with atmospheric
oxygen (Scheme 4).[7]
Scheme 3 Silanes as a Masked Hydroxy Group[6]
Scheme 4 Directed Metalation as a Means of Hydroxylation[7]
The remainder of Section 36.1 deals with the formation of alcohols from other functional groups by oxidation, reduction,
substitution, or addition reactions. Reduction of carbonyl compounds to yield primary alcohols (Section 36.1.2) is an area
where chemoselective methods offer the possibility of reducing selectively one type of carboxylic acid derivative in the
presence of another. For example, borane–tetrahydrofuran complex reduces carboxylic acids in the presence of esters,[8]
zinc(II) borohydride reduces aliphatic esters in the presence of aromatic ones,[9] and lithium triethylborohydride produces
alcohols (e.g., 8) rather than amines from amides (e.g., 7) (Scheme 5).[10]
Scheme 5 Chemoselective Synthesis of Alcohols by Reduction of Carboxylic Acid Derivatives[8–10]
Several reagents reduce aldehydes in the presence of ketones (Scheme 6),[11] while chlorodiisopinocampheylborane
exhibits remarkable selectivity for the reduction of aldehydes or ketones in the presence of acid chlorides, for example
giving alcohol 10 from aldehyde 9.[12] Temporary acetal protection in the presence of lanthanide salts provides a powerful
way of reducing ketones in the presence of aldehydes (Scheme 6).[13]
Scheme 6 Chemoselective Reduction of Aldehydes or Ketones[11–13]
Stereoselectivity also features highly in Section 36.1.2 because ketone reductions allow chiral secondary alcohols to be
produced in enantiomerically enriched form. The importance of catalytic methods for making chiral secondary alcohols on a
large scale has led to some superbly tuned catalysts, such as Noyori's family of ruthenium–diamine–diphosphine
complexes. The reduction shown in Scheme 7 generates essentially enantiomerically pure alcohol 12 in quantitative yield
from ketone 11 using sodium formate as the source of hydrogen.[14]
Scheme 7 Catalytic Asymmetric Reduction of a Ketone[14]
Substitution reactions (Section 36.1.3) allow alcohols to be formed by displacement of leaving groups with oxygen
nucleophiles, but also of course by displacement of an alcohol leaving group with other nucleophiles, as in this alkylative
substitution of acetal 13 by an aluminum reagent.[15]
Scheme 8 Alcohol Synthesis by Alkylative Substitution of an Acetal[15]
Sections 36.1.4–36.1.7 describe the synthesis of alcohols by addition to C—C and C—O multiple bonds (excluding
methods which proceed via hydroboration and oxidation, which are discussed in Section 36.1.1.3). Hydration or ozonolysis
of alkenes and alkynes form the bulk of Section 36.1.4, but also covered are connective hydroxyalkylations of unactivated
alkenes, such as the example shown in Scheme 9, using a chiral zirconocene catalyst 15. Methylation by
trimethylaluminum and air oxidation yields alcohol 16 in 74% ee from alkene 14.[16]
Scheme 9 Asymmetric Hydroxymethylation of an Alkene[16]
Carbon monoxide (Section 36.1.5) and carbon dioxide (Section 36.1.6) provide the source of a C—OH unit in the
underutilized synthesis of alcohols by carbonylation and carboxylation reactions. For example, hindered tertiary alcohols
are formed in a convergent manner by hydroboration of alkenes followed by carbonylation (Scheme 10).[17] The
formation of tertiary alcohols by multiple additions to carboxylic acid derivatives is also described in this section.
Scheme 10 Connective Synthesis of an Alcohol by Carbonylation of a Borane[17]
One of the most common C—C bond-forming reactions used in synthesis is the addition of a carbon nucleophile to an
aldehyde or a ketone, and Section 36.1.7 deals with a series of organometallic reagents in such reactions (aldol chemistry
is reserved for Section 36.9). As with ketone reduction, stereoselectivity features highly, whether diastereoselectivity, for
example in the addition of alkyllithium reagents to chiral aldehydes such as 17[18] or in the attack on cyclic ketone 18[19]
(Scheme 11), or enantioselectivity, for example in the amino alcohol promoted addition of dialkylzinc reagents (Scheme
12).[20]
Scheme 11 Synthesis of Alcohols by Diastereoselective Addition to Carbonyl Compounds[18,19]
Scheme 12 Enantioselective Addition of Diethylzinc to an Aldehyde[20]
The challenge of distinguishing the enantiotopic faces of a prochiral ketone makes tertiary alcohols particularly difficult to
prepare stereoselectively. Among the most effective methods available is the addition to thioacetals, e.g. 19, which can
subsequently be deprotected to reveal hydroxyaldehydes (Scheme 13).[21]
Scheme 13 Synthesis of a Chiral Tertiary Alcohol Using a Thioacetal Auxiliary[21]
In many cases, asymmetric synthesis is neither practical nor economical, and chiral alcohols are instead obtained by
resolution. Section 36.1.8 describes resolution methods and also methods that invert the stereochemistry at a hydroxy-
bearing center. Classical resolution techniques are less suitable for alcohols than for acids or amines, and many of the
methods described are kinetic resolutions for which a number of lipases and other enzymes work extremely well. For
example, at 51% conversion, Candida antarctica lipase converts the alcohol rac-20 into its ester 21 in 97% ee, leaving
behind unreacted alcohol (S)-20 with even higher enantiomeric purity (Scheme 14).[22]
Scheme 14 Enzymatic Kinetic Resolution of a Chiral Alcohol by Acylation[22]
The organism Corynosporium cassiicola carries out an even more spectacular resolution of trans-indane-1,2-diol (rac-22)
in which the R,R-enantiomer is simply converted, by inversion at both stereogenic centers, into the S,S-enantiomer in 82%
yield from the racemate (Scheme 15).[23]
Scheme 15 Enantiomeric Enrichment of a Diol by Corynosporium cassiicola[23]
The hydroxy group itself provides alcohols with reactivity and allows their further functionalization to generate new alcohols,
usually via another intermediate functionality. The wide variety of these transformations is explored in Section 36.1.9.
Acylations and hydroxyalkylations α to a hydroxy group can be achieved, for example, by deprotonation of an intermediate
acetal 23 (Scheme 16)[24] or carbamate 25 (Scheme 17).[25] Both processes generate enantiomerically enriched
products: the former leads to alcohol 24 by making use of a sugar-derived auxiliary, and the latter gives alcohol 26 by
employing (–)-sparteine as a chiral additive.
Scheme 16 Asymmetric α-Functionalization of an Alcohol via a Lithiated Acetal[24]
Scheme 17 Asymmetric α-Functionalization of an Alcohol via a Lithiated Carbamate[25]
Many of the methods discussed for the synthesis of alkanols in Section 36.1 are applicable to the other classes of
alcohols described in Sections 36.2–36.10. However, some have particular significance for certain classes, or exhibit
features which are relevant only in certain contexts. The cycloalkanols for example, whose synthesis is described in
Section 36.2, can all be made by simple reduction of ketones. However, cyclization methods, many applicable primarily to
a specific ring size, come into their own here. Thus, the Kulinkovich reaction is perfectly suited to cyclopropanol synthesis
(Scheme 18),[26] while samarium ketyl cyclization yields cyclobutanols (Scheme 19).[27,28] A much wider range of
intramolecular addition reactions, such as the intramolecular Prins reaction shown in Scheme 20, yield five- and
six-membered and larger rings.[29]
Scheme 18 Synthesis of a Cyclopropanol by the Kulinkovich Reaction[26]
Scheme 19 Synthesis of a Cyclobutanol via Samarium-Mediated Ketyl Cyclization[27,28]
Scheme 20 Synthesis of a Cycloalkanol via Prins Cyclization[29]
The subsequent synthetic utility of the unsaturated systems makes propargylic, allylic, and homoallylic alcohols particularly
valuable intermediates, and routes to these subclasses are dealt with in Sections 36.3, 36.5, and 36.6, respectively. Mild,
catalytic methods for C—C bond formation are of particular importance here, and many of the most important methods for
synthesizing these classes involve addition of an unsaturated alkynyl, vinyl, or allyl unit to an aldehyde or ketone, often with
control of stereochemistry. For example, the zinc-promoted addition of alkynes to aldehydes in the presence of a chiral
catalyst derived from amino alcohol 28 is one of the most simple and effective ways of making propargylic alcohols, and
with active ketones such as 27 the reaction yields tertiary alcohols enantioselectively (Scheme 21).[30]
Scheme 21 Preparation of a Tertiary Propargylic Alcohol by Alkyne Addition to a Ketone[30]
Allylic alcohols combine two of the most versatile functional groups in chemistry, and can be prepared by one of the
broadest sets of reactions imaginable, including oxidation, reduction, rearrangement, and C—C bond-forming reactions.
The latter type includes such unusual processes as the reductive alkylation of lithiated epoxides, which can be
enantioselective in the presence of (–)-sparteine as a chiral ligand.[31–33] Epoxide 29 is metalated and then couples with
isopropyllithium to yield the alkylated allylic alcohol 30 (Scheme 22). Allylic alcohols are themselves substrates for many
important transformations to other subclasses of alcohols, and feature highly in Section 36.1.9
Scheme 22 Allylic Alcohol by Desymmetrizing Reductive Alkylation of an Epoxide[31–33]
Homoallylic alcohols, covered in Section 36.6, derive primarily from the allylation of aldehydes or ketones, and their utility
lies in the fact that the double bond can itself be considered a masked carbonyl group for subsequent reactions, which
leads to 1,3-diols and hence polyketide structures. Chiral allylboron, -tin, -silicon, and -chromium (and other metal)
derivatives have been used for their synthesis, while variants which use achiral allylating agents in the presence of chiral
Lewis acids are particularly effective (Scheme 23).[34]
Scheme 23 Preparation of Homoallylic Alcohols by Asymmetric Allylation Using a Chiral Lewis Acid[34]
Homoallylic alcohols are also generated by the [2,3]-Wittig rearrangement, as in the synthetic route to the viridiofungins
shown in Scheme 24.[35]
Scheme 24 Preparation of a Homoallylic Alcohol by a [2,3]-Wittig Rearrangement[35]
Benzylic alcohols find greater interest as final targets in a synthetic sequence, and their synthesis, described in Section
36.4, may involve some reactions unique to this structural class, for example enantioselective oxidations of benzylic C—H
bonds (Scheme 25)[36] and enantioselective 1,2-Wittig rearrangements (Scheme 26).[37]
Scheme 25 Asymmetric Benzylic Oxidation[36]
Scheme 26 Synthesis of a Benzylic Alcohol by [1,2]-Wittig Rearrangement[37]
Description:New edition of the acclaimed reference series, Houben-Weyl. This new ed. is published in English and is available in both print and electronic formats. Clear and systematic, Science of Synthesis provides practical solutions and offers a route through the mass of information available in the primary