Table Of ContentEnzyme Kinetics: Catalysis & Control
A Reference of Theory
and Best-Practice Methods
This page intentionally left blank
Enzyme Kinetics:
Catalysis & Control
A Reference of Theory
and Best-Practice Methods
Daniel L. Purich
Department of Biochemistry and Molecular Biology
University of Florida College of Medicine
Gainesville, Florida
AMSTERDAM(cid:2)BOSTON(cid:2)HEIDELBERG(cid:2)LONDON(cid:2)NEWYORK(cid:2)OXFORD
PARIS(cid:2)SANDIEGO(cid:2)SANFRANCISCO(cid:2)SINGAPORE(cid:2)SYDNEY(cid:2)TOKYO
AcademicPressisanimprintofElsevier
Academic Press is animprint ofElsevier
32 JamestownRoad,London NW1 7BY,UK
30 Corporate Drive,Suite 400, Burlington, MA 01803, USA
525 BStreet, Suite 1900,SanDiego,CA 92101-4495, USA
Firstedition 2010
Copyright (cid:2) 2010Elsevier Inc. All rights reserved
No part ofthis publicationmay bereproduced, stored ina retrieval system ortransmitted inanyform orby anymeans
electronic, mechanical, photocopying, recording or otherwisewithoutthe priorwritten permissionof the publisher.
PermissionsmaybesoughtdirectlyfromElsevier’sScience&TechnologyRightsDepartmentinOxford,UK:phone(+44)
(0) 1865843830;fax (+44) (0) 1865853333;email: [email protected]. Alternatively, visit the Science and
Technology Books website at www.elsevierdirect.com/rights for further information
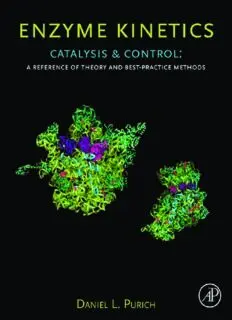
CoverImage:Twoviews of the X-ray crystallographic structure ofthe completeThermusthermophilus 70S ribosome
containing boundmessenger RNAand transferRNAs at 5.5-A˚ resolution (from Yusupov, M.M., Yusupova,G. Z.,
Baucom, A.,Lieberman, K.,Earnest, T. N.,Cate, J. H.,and Noller,H. F. (2001) Science 292, 883–96 with permission).
PerhapsNatures’smostcomplicatedmolecularmachine,theribosomeconsistsofRNAandproteinsthatworktogetherto
accomplishthe multiple structural,catalytic,and force-generating steps requiredfor the high-fidelitysynthesis and
elongationofpolypeptides.Althoughthecenterpieceofthe2009NobelPrizesinChemistry,theribosomeremainsamajor
challenge for enzyme scientists and kineticists seekingto unlock itsmanysecrets.
Notice
No responsibilityis assumed by the publisher for anyinjury and/ordamage topersonsor propertyas amatterof
productsliability, negligenceorotherwise, orfrom anyuseoroperation ofanymethods, products,instructions or
ideascontained inthe materialherein.Becauseof rapid advances inthe medical sciences, inparticular, independent
verification of diagnoses anddrug dosagesshouldbe made
British Library Cataloguing-in-PublicationData
A catalogue record for this bookis available from the British Library
Libraryof CongressCataloging-in-Publication Data
A catalog recordfor this book isavailable from the Library ofCongress
ISBN:978-0-12-380924-7
For information on all Academic Press publications
visitourwebsiteatelsevierdirect.com
Typeset byTNQ Books andJournals PvtLtd.
www.tnq.co.in
Printed and bound inChina
10 11 12 13 1415 10 9 87 6 5 43 2 1
Contents
Table of Contents
Preface xix toUnderstandtheDetailedMutual
ChangesinBothSubstrate
andEnzymeDuringCatalysis 34
1.6.2. WeNeedNewApproaches
forDeterminingthe
Chapter 1. An Introduction to Enzyme
ChannelsAllowingEnergy
Science 1
FlowDuringEnzyme
1.1. Catalysis 5 Catalysis 37
1.1.1. RootsofCatalysisinthe 1.6.3. WeNeedAdditionalProbes
EarliestChemicalSciences 5 ofEnzymeCatalysis 38
1.1.2. SyntheticCatalysts 7 1.6.4. WeNeedtoLearnHow
1.2. BiologicalCatalysis 12 ProteinsFoldandHowto
1.2.1. RootsofEnzymeScience 12 ManipulateProteinStability 38
1.2.2. EnzymeTechnology 13 1.6.5. WeNeedtoDevelopa
1.3. DevelopmentofEnzymeKinetics 15 DeeperUnderstandingof
1.4. TheConceptofaReactionMechanism 19 SubstrateSpecificity 39
1.4.1. Chymotrypsin:ThePrototypical 1.6.6. WeNeedtoDevelopthe
BiologicalCatalyst 20 AbilitytoDesignEntirely
1.4.2. Ribozymes 22 NewBiologicalCatalysts 42
1.4.3. Mechanoenzymes 23 1.6.7. WeNeedtoDefinethe
1.5. ExplainingtheEfficiencyofEnzyme EfficientRoutesforObtaining
Catalysis 25 HighPotencyEnzymeInhibitors
1.5.1. StabilizationofReaction asDrugsandPesticides 44
TransitionStates 26 1.6.8. WeNeedtoLearnMore
1.5.2. ElectrostaticStabilization AboutInSinguloEnzyme
ofTransitionStates 27 Catalysis 45
1.5.3. IntrinsicBindingEnergy 28 1.6.9. WeNeedtoDevelop
1.5.4. ReactingGroup ComprehensiveCatalogs
Approximation,Orientation ofEnzymeMechanismsand
andOrbitalSteering 28 toUseSuchInformationin
1.5.5. ReactantStateDestabilization 29 FashioningNewMetabolic
1.5.6. Acid/BaseCatalysis 30 Pathways 46
1.5.7. CovalentCatalysis 30 1.6.10. WeNeedtoUnderstand
1.5.8. Transition-StateStabilization HowtoAnalyzetheKinetic
byLow-BarrierHydrogen BehaviorofDiscrete
Bonds 31 Enzyme-CatalyzedReactions
1.5.9. CatalyticFacilitationby asWellasMetabolic
MetalIons 32 PathwaysintheirEnvironment 48
1.5.10. PromotionofCatalysisvia 1.6.11. WeNeedtoDevelop
EnzymeConformational TechniquesthatwillFacilitate
Flexibility 32 InvestigationofChromosomal
1.5.11. PromotionofCatalysis Remodeling,Epigenetics,and
viaForce-Sensingand theGeneticBasisof
Force-GatedMechanisms 33 DiseaseandCellSurvival 49
1.6. ProspectsforEnzymeScience 34 1.6.12. WeNeedtoDevelop
1.6.1. WeNeedBetterMethodsfor EffectiveEnzymePreparationsfor
AnalyzingEnzymeDynamics UseinDirectEnzymeTherapy 50
v
Contents
vi
Chapter 2. Active Sites and their Chemical 2.4.2. SomeEnzymesExploit
SpecializedAmino-Acid
Properties 53
ResiduesinCatalysis 78
2.1. EnzymeActiveSites 54 2.5. MetalIonsinEnzymeActiveSites 81
2.1.1. MostEnzymesareProteins, 2.5.1. AGroupofBiologically
whichareLinearPolymersof SignificantMetalIonsis
a-AminoCarboxylicAcids 55 EssentialforCatalysis
2.1.2. Active-SiteResiduesmaybe bySomeEnzymes 82
ClassifiedwithRespectto 2.5.2. Enzyme-BoundMetalIon
theirFunction(s) 57 ComplexesShareStructural
2.1.3. ActiveSitesTypicallyOccupy andChemicalFeatures 84
only2–3percentoftheTotal 2.5.3. TheChemistryofMetal
VolumeofanEnzyme 59 Ion-LigandComplexesis
2.1.4. BindingEnergyOftenIndicates DominatedbytheNatureof
theStrengthofEnzyme theirLigancy 85
InteractionswithSubstrates 2.5.4. Field-EffectsInfluencethe
andCofactors 60 ColorandMagnetic
2.1.5. TheStructuralOrganization PropertiesofMetalIon
ofEnzymescanbeConsidered CoordinationComplexes 87
Hierarchically 61 2.5.5. TheReactionMechanismsof
2.1.6. EnzymesOftenOccurin TransitionMetalComplexes
MultipleMolecularForms 63 areDeterminedbytheir
2.2. ForcesAffectingEnzymeStructural Inner-andOuter-Sphere
StabilityandInteractions 64 CoordinationBehavior 89
2.2.1. ElectrostaticInteractions 2.5.6. MetalIonsFormComplexes
InfluenceEnzymeStructure withEnzymesand/ortheir
andInteractions 64 Substrates 93
2.2.2. Ion–DipoleandDipole–Dipole 2.5.7. PropertiesofSelected
InteractionsareSpecialized Active-SiteMetalIons 95
ElectrostaticPhenomena 66 2.5.8. ASurveyofMetalIonComplexes
2.2.3. HydrogenBondingMainly withinSelectedEnzymesReveals
PlaysaCompensatoryRole KeyFeaturesofBinding-Site
inStabilizingProteins 66 Organization 109
2.2.4. HydrophobicInteractions
2.6. ActiveSitesofEnzymesActingon
PlayaDominantRolein
PolymericSubstrates 112
StabilizingMostProteins 69 2.6.1. ManyEndonucleasesAchievetheir
2.2.5. AlthoughIndividuallyWeak, RemarkableSpecificitybyMeans
vanderWaalsInteractions ofSubsiteRecognition 113
aresoNumerousthatthey 2.6.2. ProteasesweretheFirstEnzymes
ContributeSignificantly ShowntohaveSubsitesforInteracting
toOverallProteinStability 70 withtheirPolymericSubstrates 114
2.2.6. SomeProteinsare 2.6.3. Endo-Glycosidasesalso
OccasionallyStabilizedby ExploitSubsitestoAchieveSpecificity 115
p-CationInteractions 70 2.6.4. SubsitesFacilitateSubstrate
2.3. Active-SiteDiversification 70 RecognitionbySignal-
2.3.1. EnzymeDiversificationcan TransducingProteinKinases 115
beExplainedStructurally 71
2.7. BasicOrganicChemistryofEnzyme
2.3.2. CatalyticPromiscuitymay
Action 116
ExplaintheEmergenceof 2.7.1. ThereareSixMajorClassesof
CatalyticallyDiversified Enzyme-CatalyzedCovalent
Enzymes 74 Bond-Making/-Breaking
2.4. AdditionalFunctionalGroupsin Reactions 118
EnzymeActiveSites 77 2.7.2. CarbonhasSeveralReactive
2.4.1. Vitamin-BasedCoenzymesIncrease FormsinEnzymaticMechanisms 119
theChemicalVersatilityofEnzyme 2.7.3. ManyEnzymesUsetheSame
ActiveSites 77 GeneralReactionMechanisms
Contents
vii
FirstDiscoveredbyPhysical CoordinatedElectron
OrganicChemists 120 TransferReactions 158
2.7.4. NucleophilicSubstitutionisa 2.10.4. Enzyme-CatalyzedElectron
WidelyUsedReaction Transfermaybeanalyzed
MechanisminEnzymeCatalysis 121 byMarcusTheory 160
2.7.5. Enzyme-CatalyzedElimination 2.10.5. SimpleKineticModelscan
ReactionMechanismshaveMany AccountfortheBehaviorof
PrecedentsinOrganicChemistry 125 BiologicalElectronTransferReactions 161
2.7.6. EnzymesareHighlyEffectivein 2.10.6. SeveralPrototypicalRedox
Forming,Stabilizing,and EnzymesProvideValuable
UtilizingCarbanion InsightsintoElectronTransfer
IntermediatesDuringCatalysis 125 KineticsandMechanisms 162
2.7.7. FreeRadicalsareFormedin 2.10.7. EnzymeElectrodesCombine
aSurprisingNumberof theSpecificityofBiological
Enzyme-CatalyzedReactions 131 CatalysiswiththeVersatilityof
2.7.8. TheVersatilityofEnzymescan PotentiometryorAmperometry 164
beIllustratedbyConsidering Appendix 168
aSelectedGroupofReaction
Mechanisms 134
2.8. DetectingCovalentIntermediatesin
Chapter 3. Fundamentals of Chemical
EnzymeReactions 137
2.8.1. EnzymesFormaWideRange Kinetics 171
ofEnzyme-SubstrateCovalent
3.1. TimescaleofChemicalProcesses 171
Compounds,andManyare
3.2. TheEmpiricalRateEquation 172
CatalyticallyCompetent 137
3.3. ReactionRate,OrderandMolecularity 174
2.8.2. Side-ReactionsOftenProvide
3.3.1. ReactionRate 174
InvaluableCluesAbout
3.3.2. ReactionOrder 175
MechanismsofEnzyme
3.3.3. Molecularity 176
Catalysis 141
3.3.4. Zero-OrderKinetics 177
2.8.3. SomeEnzyme-Substrate
3.3.5. First-OrderKinetics 177
CovalentCompoundscanbe
3.3.6. Second-OrderKinetics 180
ChemicallyTrapped 143
3.3.7. PseudoFirst-OrderKinetics 180
2.9. BasicsofEnzymeStereochemistry 145
3.4. BasicStrategiesforEvaluatingRateProcesses 181
2.9.1. Definitions 145
3.4.1. Initial-RateMethod 181
2.9.2. TheCahn-Ingold-PrelogSystem
3.4.2. ProgressCurveAnalysis 182
AllowsOnetoAssigntheAbsolute
3.5. CompositeMulti-stage(Multi-step)
StereochemicalConfiguration
Mechanisms 184
ofChiralCompounds 146
3.5.1. SeriesFirst-OrderKinetics 185
2.9.3. TheProchiralityofMolecules
3.5.2. ReversibleFirst-OrderKinetics 186
mayalsobeSpecified
3.5.3. ReversibleSecond-OrderKinetics 186
Systematically 147
3.5.4. Rapid-Equilibriumand
2.9.4. TheStereochemistryofMethyl
Steady-StateTreatments 186
TransferReactionsmaybe
3.5.5. Rate-ControllingSteps 188
AnalyzedUsingEnzymesof
3.5.6. PrinciplesofDetailedBalance 189
KnownStereochemistry
3.5.7. ThermodynamicCyclesfor
asReferenceReactions 149
EvaluatingDetailedBalance 190
2.10. ElectronTransferReactions 150 3.5.8. KineticEquivalenceand
2.10.1. TheThermodynamicProperties
MechanisticAmbiguity 192
ofOxidation–Reduction
3.6. ThermalEnergy:TheBoltzmann
ReactionsareDefinedby
DistributionLaw 192
RedoxPotentials 154
3.7. SolutionBehaviorofReacting
2.10.2. TheRedoxBehaviorofComplex
Molecules 194
Metalloenzymescanbe
3.7.1. Water:AUniqueSolventfor
EvaluatedSpectroscopically
BiochemicalProcesses 194
byStoichiometricTitrationTechniques 157
3.7.2. DiffusionLimitationson
2.10.3. RespiratoryChainsare
ChemicalProcessesOccurring
ComprisedofHighly
inWater 196
Contents
viii
3.7.3. ElectrostaticEffectson 4.4.2. Beer’sLawisaQuantitative
MagnitudeofBimolecular ExpressionLinkingAbsorbance
RateConstants 199 toConcentration 240
3.7.4. ReactantDesolvation 200 4.4.3. SomeEnzymeAssaysUse
3.8. Transition-StateTheory 201 AlternativeSubstratesthatare
3.9. ChemicalCatalysis 203 Chromogenic 243
3.9.1. AcceleratingRatewithout 4.5. BasicFluorescenceSpectroscopy 243
AlteringtheEquilibriumPoise 203 4.5.1. FluorescenceSpectraDepend
3.9.2. NucleophilicandElectrophilic onExcited-StateRelaxation 244
Facilitation 205 4.5.2. FeaturesofaResearch-Grade
3.9.3. BufferCatalysis 206 Spectrophotofluorimeter 244
3.9.4. Autocatalysis 206 4.5.3. TheConcentrationofVarious
3.10. ReactionCoordinateDiagrams 207 MetabolitesmaybeQuantified
3.11. ThermodynamicPrinciples 210 ThroughFluorescence
3.11.1. ChemicalEquilibrium 210 Spectrometry 246
3.11.2. DirectionandExtentof 4.5.4. BiologicalMoleculesmay
ChemicalReaction 210 ContainIntrinsicorExtrinsic
3.11.3. UsingDDGtoDefineBinding FluorescentReporterGroups 247
Energetics 211 4.5.5. FluorescenceAnisotropyisa
3.11.4. AlbertyTreatmentof PowerfulTechniqueForQuantifying
BiochemicalThermodynamics 211 BindingInteractions 250
3.11.5. SomeReactingSystemsare 4.5.6. Fo¨rster(Fluorescence)Resonance
BestAnalyzedbyPrinciples EnergyTransfer(FRET)isan
ofNon-Equilibrium ExquisitelyDistance-Sensitive
Thermodynamics 212 ProbeofEnzymes 252
3.12. ConcludingRemarks 214 4.5.7. ContinuousFluorescenceAssays
areNowAvailableforPi-and
PPi-ProducingReactions 253
Chapter 4. Practical Aspects of Measuring 4.5.8. Chemiluminescenceisa
Initial Rates and Reaction PhotoemissiveProcessOften
ExploitedinEnzymeRateAssays 254
Parameters 215
4.6. MeasuringReactionRateswithIsotopes 255
4.1. DesignofInitial-VelocityEnzymeAssays 215 4.6.1. StableIsotopesareVersatile
4.1.1. ‘‘ActivityPurity’’isSufficientin ProbesinEnzymeKinetics 255
MostInitial-RateStudies 216 4.6.2. RadioisotopesProvideExtremely
4.1.2. DiscontinuousandContinuous SensitiveAssaysofEnzyme
RateMeasurements 217 RateProcesses 260
4.1.3. EachEnzymeRateAssayhasIts
4.7. MultisubstrateKineticsandInhibitor
OwnSpecialSetofRequirements 220
Kinetics 264
4.2. EnzymePurification 232 4.8. AnalysisofEnzymeRateData 265
4.2.1. WhileTime-Consuming,theTask 4.8.1. EnzymeRateDatamustbe
ofEnzymePurificationisOften AppropriatelyWeighted 266
WellFounded 232 4.8.2. QuantitativeAnalysisofReaction
4.2.2. BiochemistshaveDevelopeda Progress-CurvescanbeUsedto
PowerfulBatteryofTechniques EvaluateRateParameters 268
forPurifyingEnzymes 234 4.8.3. GlobalAnalysisOffersAdded
4.3. Coupled(orAuxiliary)EnzymeAssays 235 AdvantagesinStatisticalAnalysis 270
4.3.1. ASimpleKineticTreatment
4.9. WorkingwithATP-DependentEnzymes 272
ExplainstheLag-Phasein
4.10. RegeneratingNucleoside
CoupledEnzymeAssays 238 59-TriphosphateSubstrates 275
4.3.2. TheAuxiliaryEnzymeandAssay 4.10.1. ProteinandEnzyme
ConditionsmustbeSuitedto Concentration 276
thePrimaryEnzymeReaction 239 4.10.2. TotalProteinConcentration
4.4. BasicUV/VisibleAbsorption canbeDeterminedQuantitatively 276
Spectroscopy 240 4.10.3. ActiveEnzymeConcentrationcan
4.4.1. AbsorptionSpectraDependonthe beQuantifiedbySeveralTechniques 276
QuantumStatesofElectronOrbitals 240
Contents
ix
4.11. EquilibriumConstantDeterminations 278 5.3. CatalysisInvolvingTwoorMore
4.11.1. EquilibriumConstantscanbe Intermediates 298
EvaluatedinaVarietyofWays 279 5.3.1. DerivationoftheTwo-
4.11.2. TheDeterminationofthe IntermediateCaseIllustrates
ArginineKinaseReaction WhythisTreatmentisaMore
EquilibriumConstantisan RealisticRepresentation
ExcellentExampleofa ofanEnzymeMechanism 298
Well-Designedand 5.3.2. AShortcutcanbeTakento
Well-ExecutedDetermination 281 DerivetheSteady-StateRate
4.12. ConcludingRemarks 284 EquationfortheReverse
Two-IntermediateReaction
Scheme 298
5.3.3. MultipleInternalIsomerizationsare
Chapter 5. Initial-Rate Kinetics of One-
withoutEffectontheGeneral
Substrate Enzyme-Catalyzed
FormoftheFinalSteady-State
Reactions 287 RateEquation 298
5.3.4. TheHaldaneRelationship
5.1. Michaelis-MentenTreatment 287
alsoConstrainstheRelative
5.1.1. DerivationoftheMichaelis-
MagnitudesofKeyRate
MentenEquationRevealshow
ParametersintheTwo-
KeyAssumptionsDefinean
IntermediateScheme 300
Enzyme’sInitial-RateBehavior 288
5.1.2. KS,Vm,Vm/KS,and[S]/KSare 5.4. AdditionalCommentsonFundamental
RateParametersDefiningan KineticParameters 301
Enzyme’sInitial-RateBehavior 289 5.4.1. TheMichaelisConstanthas
SeveralImportantImplications,
5.1.3. SeveralMethodsforPlottingInitial-
withRegardtoBoth‘‘Substrate
RateDataareQuiteUsefulbuthave
Affinity’’andSubstrate
InherentLimitations 290
Specificity 301
5.1.4. TheMichaelis-MentenEquation
PredictsaLinearDependence 5.4.2. TheTurnoverNumberkcat
IndicatesNumberofSubstrate
ofReactionRateonthe
MoleculesConvertedto
ConcentrationofActiveEnzyme 292
ProductperEnzymeActive
5.1.5. TheQuadraticFormulais
SiteperSecond 303
RequiredWhentheEnzyme
ConcentrationApproaches 5.4.3. The‘‘SpecificityConstant’’
SubstrateConcentration 292 Vmax/Kmorkcat/KmIndicatesthe
EfficiencyofSubstrateCapture
5.1.6. NonproductiveSubstrateBinding
byanEnzyme 304
CannotbeDetectedbytheMichaelis-
MentenTreatment 293 5.4.4. TheCommitmenttoCatalysis
MeasuresanEnzyme’sAbility
5.2. TheBriggs-haldaneSteady-State
toConverttheE$SComplex
Treatment 293
toE$P,asComparedto
5.2.1. DerivationofthisRateEquation
ReconversionofE$StoaPrior
RevealsKeyFeaturesof
EnzymeForm 307
Steady-StateProcesses 294
5.4.5. EvolutionofCatalyticProficiency 308
5.2.2. ReactionEnergeticsDetermine
5.4.6. InternalEquilibriaand
theEffectofIncreasingSubstrate
EnergeticsofPerfectedEnzymes 309
ConcentrationontheConversion
ofEþStoE$SComplex 295 5.5. ReactionProgressCurveAnalysis 310
5.2.3. TheCorrespondingReverse-Reaction 5.6. RibozymeKinetics 311
RateEquationcannowbeWritten 295 5.7. ProteasomeKinetics 313
5.2.4. TheHaldaneRelationship 5.8. IsomerizationMechanisms 314
ConstrainstheValuesofKey 5.9. SimultaneousActionofanEnzyme
RateParametersforReversible onDifferentSubstrates 315
Enzyme-CatalyzedReactions 296 5.10. EnantiomericEnrichmentand
5.2.5. TheBriggs-HaldaneEquation AnomericSpecificity 316
RequiresthatanEnzyme 5.11. SimultaneousActionofTwoEnzymes
SystemSatisfiestheSteady- ontheSameSubstrate 318
StateAssumption 296 5.12. Induced-FitMechanisms 319