Table Of Content(cid:6)(cid:13)(cid:13)(cid:14)(cid:14)(cid:14)(cid:14)(cid:4)(cid:4)(cid:15)(cid:15)(cid:16)(cid:16)(cid:2)(cid:2)(cid:11)(cid:11)(cid:15)(cid:15)(cid:7)(cid:7)(cid:10)(cid:10)(cid:12)(cid:12)(cid:17)(cid:17)(cid:6)(cid:6)(cid:18)(cid:18)(cid:19)(cid:19)(cid:5)(cid:5)(cid:5)(cid:5)(cid:6)(cid:6)(cid:20)(cid:20)(cid:2)(cid:2)(cid:21)(cid:21)(cid:15)(cid:15)(cid:16)(cid:16)(cid:2)(cid:2)(cid:4)(cid:4)(cid:6)(cid:6)(cid:22)(cid:22)(cid:10)(cid:10)(cid:15)(cid:15)(cid:11)(cid:11)(cid:15)(cid:15)(cid:2)(cid:2)(cid:11)(cid:11)(cid:7)(cid:7)(cid:19)(cid:19)(cid:12)(cid:12)
Photoinitiators: Classification
A photoinitiator is a compound especially added to a (cid:127) Type II photoinitiators undergo a bimolecular reaction
formulation to convert absorbed light energy, UV or visible where the excited state of the photoinitiator interacts
light, into chemical energy in the form of initiating species, with a second molecule (a coinitiator) to generate free
viz., free radicals or cations. Based on the mechanism by radicals.
which initiating radicals are formed, photoinitiators are
generally divided into two classes: UV photoinitiators of both Type I and Type II are available.
However, visible light photoinitiators belong almost
(cid:127) Type I photoinitiators undergo a unimolecular bond cleav- exclusively to the Type II class of photoinitiators. Table I
age upon irradiation to yield free radicals. summarizes the various classes of available Type I and Type
II photoinitiators and their common applications.
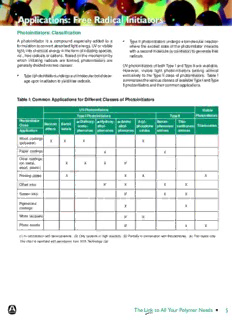
Table I: Common Applications for Different Classes of Photoinitiators
UV-Photoinitiators Visible
Type I Photoinitiators Type II Photoinitiators
Photoinitiator aaaaa-Dialkoxy- aaaaa-Hydroxy- aaaaa-Amino Acyl- Benzo- Thio-
Benzoin Benzil
Class aceto- alkyl- alkyl- phosphine phenones/ xanthones/ Titanocenes
ethers ketals
Application phenones phenones phenones oxides amines amines
Wood coatings
X X X X
(polyester)
Paper coatings X X
Clear coatings
(on metal, X X X X2
wood, plastic)
Printing plates X X X X
Offset inks X1 X X X
Screen inks X3 X X
Pigmented
X X
coatings
White lacquers X4 X
Photo resists X3 X X
(1) In combination with benzophenone. (2) Only systems of high reactivity. (3) Partially in combination with thioxanthones. (4) Thin layers only.
This chart is reprinted with permission from SITA Technology Ltd.
a (cid:19)(cid:17)(cid:6)(cid:8)(cid:20)(cid:16)(cid:21)(cid:22)(cid:8)(cid:12)(cid:2)(cid:8)(cid:15)(cid:3)(cid:3)(cid:8)(cid:23)(cid:2)(cid:10)(cid:7)(cid:8)(cid:1)(cid:2)(cid:3)(cid:4)(cid:5)(cid:6)(cid:7)(cid:8)(cid:24)(cid:6)(cid:6)(cid:9)(cid:13) (cid:1) (cid:27)
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra
For photoinitiation to proceed efficiently, the absorption bands additional substituted acetophenones and benzophenones
of the photoinitiator must overlap with the emission that are potential photoinitiators are available from Aldrich;
spectrum of the source and there must be minimal search our product database at www.sigma-aldrich.com.
competing absorption by the components of the formulation
at the wavelengths corresponding to photoinitiator Spectra were recorded on a Perkin Elmer UV/Vis Lambda 2
excitation. For the initial selection of a photoinitiator in your spectrophotometer using Perkin Elmer Computerized
application, viz., one with excitation wavelengths that lie in Spectroscopy Software 4.01. Concentrations are expressed
the emission spectrum of your UV source, as well as in the as % weight of solute in volume of solvent. The solvent
absorption window of your formulation, information about the used was A.C.S. spectrophotometric grade methyl alcohol.
photoinitiator absorption spectrum is essential. For comparison purposes, the emission spectrum of a
medium pressure mercury lamp, the most widely used
To aid in this initial selection, UV absorption spectra of 47 energy source, is shown as the last figure.
commonly used radical and cationic photoinitiators (see
Table I) are displayed in this section. Approximately 100
Table I: Commonly Used Radical and Cationic Photoinitiators whose UV Absorption Spectra are
Displayed in this Section. (Click on the photoinitiator name coded red to view the UV
spectrum. Click on the product number coded blue to link to the product document sheet
on the Sigma-Aldrich Web site.)
Cat. No. Photoinitiator Cat. No. Photoinitiator
A1,070-1 Acetophenone, 99% 19,611-8 2,2-Dimethoxy-2-phenylacetophenone, 99%
A8,840-9 Anisoin, 95% 14,934-9 4-(Dimethylamino)benzophenone, 98%
A9,000-4 Anthraquinone, 97% 14,670-6 4,4'-Dimethylbenzil, 97%
12,324-2 Anthraquinone-2-sulfonic acid, sodium salt D14,966-7 2,5-Dimethylbenzophenone, tech., 95%
monohydrate, 97% D14,967-5 3,4-Dimethylbenzophenone, 99%
11,931-8 (Benzene) tricarbonylchromium, 98% 40,566-3 Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide/
B515-1 Benzil, 98% 2-Hydroxy-2-methylpropiophenone, 50/50 blend
39,939-6 Benzoin, sublimed, 99.5+% 27,571-9 4'-Ethoxyacetophenone, 98%
17,200-6 Benzoin ethyl ether, 99% E1,220-6 2-Ethylanthraquinone, 97+%
19,578-2 Benzoin isobutyl ether, tech., 90% F40-8 Ferrocene, 98%
B870-3 Benzoin methyl ether, 96% 32,810-3 3'-Hydroxyacetophenone, 99+%
B930-0 Benzophenone, 99% 27,856-4 4'-Hydroxyacetophenone, 99%
40,562-0 Benzophenone/1-Hydroxycyclohexyl phenyl 22,043-4 3-Hydroxybenzophenone, 99%
ketone, 50/50 blend H2,020-2 4-Hydroxybenzophenone, 98%
26,246-3 3,3',4,4'-Benzophenonetetracarboxylic 40,561-2 1-Hydroxycyclohexyl phenyl ketone, 99%
dianhydride, sublimed, 98% 40,565-5 2-Hydroxy-2-methylpropiophenone, 97%
B1,260-1 4-Benzoylbiphenyl, 99% 15,753-8 2-Methylbenzophenone, 98%
40,564-7 2-Benzyl-2-(dimethylamino)-4'- 19,805-6 3-Methylbenzophenone, 99%
morpholinobutyrophenone, 97% M3,050-7 Methybenzoylformate, 98%
16,032-6 4,4'-Bis(diethylamino)benzophenone, 99+% 40563-9 2-Methyl-4'-(methylthio)-2-morpholinopropio-
14,783-4 4,4'-Bis(dimethylamino)benzophenone, 98% phenone, 98%
12,489-3 Camphorquinone, 98% 15,650-7 Phenanthrenequinone, 99+%
C7,240-4 2-Chlorothioxanthen-9-one, 98% 29,074-2 4'-Phenoxyacetophenone, 98%
40,807-7 (Cumene)cyclopentadienyliron(II) T3,400-2 Thioxanthen-9-one, 98%
hexafluorophosphate, 98% 40,722-4 Triarylsulfonium hexafluoroantimonate salts,
D3,173-7 Dibenzosuberenone, 97% mixed, 50% in propylene carbonate
22,710-2 2,2-Diethoxyacetophenone, 95% 40,721-6 Triarylsulfonium hexafluorophosphate salts,
D11,050-7 4,4'-Dihydroxybenzophenone, 99% mixed, 50% in propylene carbonate
6 • Polymer Products from Aldrich a
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra (continued)
A1,070-1 Acetophenone, 99% A8,840-9 Anisoin, 95%
O
C CH3 O
CH3O CH C OCH3
OH
0.0224 % 0.0040 %
0.0011 % 0.0014 %
12,324-2 Anthraquinone-2-sulfonic acid, sodium salt
A9,000-4 Anthraquinone, 97%
monohydrate, 97%
O O O
S ONa
O H2O
O
O
0.0010% 0.0033 %
0.0004 % 0.0006 %
a The Link to All Your Polymer Needs • 7
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra (continued)
11,931-8 (Benzene) tricarbonylchromium , 98% B515-1 Benzil, 98%
1
O O
.8 Cr C C
OC CO
CO
.8
.6
0.0023 % 0.0029 %
.6
nce 0.0012 % nce 0.0011 %
ba ba
or or
s s
b b
A .4 A
.4
.2
.2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
39,939-6 Benzoin, sublimed, 99.5+% 17,200-6 Benzoin ethyl ether, 99%
O O
CH C CH C
.8 .8
OH OCH2CH3
.6 0.0056 % .6 0.0027 %
nce 0.0017 % nce 0.0019 %
ba ba
or or
Abs Abs
.4 .4
.2 .2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
8 • Polymer Products from Aldrich a
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra (continued)
19,578-2 Benzoin isobutyl ether, tech., 90% B870-3 Benzoin methyl ether, 96%
O O
.8 CH C CH C
OCH2CHCH3 .8 OCH3
CH3
.6
0.0110 % .6 0.0043 %
nce 0.0069 % nce 0.0020 %
ba ba
or or
bs bs
A .4 A
.4
.2
.2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
40,562-0 Benzophenone/1-Hydroxycyclohexyl
B930-0 Benzophenone, 99%
phenyl ketone, 50/50 blend
O O
.8 C C
.8
O
C
OH
.6 0.0030 % .6 0.0150 %
ce 0.0012 % ce 0.0015 %
n n
a a
b b
or or
s s
b b
A A
.4 .4
.2 .2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
a The Link to All Your Polymer Needs • 9
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra (continued)
26,246-3 3,3',4,4'-Benzophenonetetracarboxylic
B1,260-1 4-Benzoylbiphenyl, 99%
dianhydride, sublimed, 98%
O O O
.8 O O O C
O C O
.6
0.0030 % 0.0031 %
ce 0.0010 % 0.0004 %
n
a
b
or
s
b
A
.4
.2
0
220 240 260 280 300 320 340 360 380 400 420 440
Nanometers
40,564-7 2-Benzyl-2-(dimethylamino)-4'-
16,032-6 4,4'-Bis(diethylamino)benzophenone, 99+%
morpholinobutyrophenone, 97%
O CH2
.8 O N C C N(CH3)2 .8
CH2CH3
.6 0.0035 % .6 0.0020 %
ce 0.0020 % ce 0.0008 %
n n
a a
b b
or or
s s
b b
A A
.4 .4
.2 .2
CH3CH2 O CH2CH3
CH3CH2N C NCH2CH3
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
10 • Polymer Products from Aldrich a
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra (continued)
14,783-4 4,4'-Bis(dimethylamino)benzophenone, 98% 12,489-3 Camphorquinone, 98%
CH3 CH3
.8 .8 R O R = CH3 R =H or
R = H R =CH3
R
O
.6 0.0031% .6 0.0271 %
ce 0.0008 % ce 0.0071 %
n n
a a
b b
or or
s s
b b
A A
.4 .4
.2 .2
CH3 O CH3
N C N
CH3 CH3
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
40,807-7 (Cumene)cyclopentadienyliron(II)
C7,240-4 2-Chlorothioxanthen-9-one, 98%
hexafluorophosphate, 98%
O
Cl
Fe
.8 S .8 CH3 PF6
CHCH3
.6 0.0024 % .6 0.0055 %
nce 0.0007 % nce 0.0027 %
ba ba
or or
bs bs
A A
.4 .4
.2 .2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
a The Link to All Your Polymer Needs • 11
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra (continued)
D3,173-7 Dibenzosuberenone, 97% 22,710-2 2,2-Diethoxyacetophenone, 95%
O OCH2CH3
C CH
.8 O .8 OCH2CH3
.6 0.0016 % .6 0.0100 %
Absorbance 0.0005 % Absorbance 0.0014 %
.4 .4
.2 .2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
D11,050-74,4'-Dihydroxybenzophenone, 99% 19,611-8 2,2-Dimethoxy-2-phenylacetophenone, 99%
1
O O OCH3
HO C OH C C
OCH3
.8 .8
0.0036 % 0.0020 %
.6 .6
nce 0.0009 % nce 0.0011 %
ba ba
or or
bs bs
A A
.4 .4
.2 .2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
12 • Polymer Products from Aldrich a
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra (continued)
14,934-9 4-(Dimethylamino)benzophenone, 98% 14,670-6 4,4'-Dimethylbenzil, 97%
1
O O
.8 CH3 C C CH3
.8
.6 0.0043 % 0.0030 %
.6
nce 0.0009 % nce 0.0010 %
ba ba
or or
bs bs
A A
.4
.4
.2 .2
CH3 O
N C
CH3
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
D14,966-7 2,5-Dimethylbenzophenone, tech., 95% D14,967-5 3,4-Dimethylbenzophenone, 99%
O
CH3 O CH3 C
.8 C .8 CH3
CH3
.6
.6
0.0296 % 0.0039 %
nce 0.0015 % nce 0.0013 %
ba ba
or or
bs bs
A .4 A .4
.2 .2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
a The Link to All Your Polymer Needs • 13
AApppplliiccaattiioonnss:: FFrreeee RRaaddiiccaall IInniittiiaattoorrss
Photoinitiators: UV Absorption Spectra (continued)
40,566-3 Diphenyl(2,4,6 trimethylbenzoyl)phosphine oxide
27,571-9 4'-Ethoxyacetophenone, 98%
2-Hydroxy-2-methylpropiophenone 50/50 blend
CH3O O
.8 CH3 C P O
CH3 .8 C CH3
O OH OCH2CH3
.6 C C CH3
CH3
0.0223 % .6 0.0044 %
Absorbance .4 0.0019 % Absorbance 0.0009 %
.4
.2
.2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
E1,220-6 Ethylanthraquinone, 97+% F40-8 Ferrocene, 98%
1
O
Fe
CH2CH3
.8
.8 O
0.0018 % .6 0.0065 %
.6
ce 0.0005 % ce 0.0028 %
n n
a a
b b
or or
s s
b b
A A
.4
.4
.2 .2
0 0
220 240 260 280 300 320 340 360 380 400 420 440 220 240 260 280 300 320 340 360 380 400 420 440
Nanometers Nanometers
14 • Polymer Products from Aldrich a
Description:Photoinitiators a-Dialkoxy- a-Hydroxy- a-Amino. Acyl-. Benzo-. Thio-. Benzoin Benzil aceto- alkyl- alkyl- phosphine phenones/ xanthones/ Titanocenes.