The taxonomy and biogeography of the exhausta group of the genus Baeturia Stål, 1866 (Homoptera, Tibicinidae) PDF
Preview The taxonomy and biogeography of the exhausta group of the genus Baeturia Stål, 1866 (Homoptera, Tibicinidae)
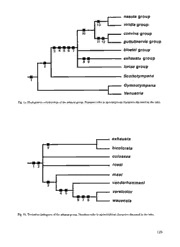
Beaufortia INSTITUTE FOR SYSTEMATICSANDPOPULATIONBIOLOGY UNIVERSITYOFAMSTERDAM Vol. 44, no.5 November 18, 1994 Thetaxonomyandbiogeographyof theexhaustagroupofthegenus Baeturia Stål, 1866(Homoptera, Tibicinidae) A.J. deBoer InstituteforSystematicsandPopulation Biology (Zbdlogisch Museum), University ofAmsterdam, POBox 94766, 1090GT Amsterdam, TheNetherlands Abstract The exhaustagroup is proposed for asupposedly monophyletic group ofeightspecies ofthe cicada genus BaeturiaStal, 1866. Three species (B. bicolorata Distant, B. exhaustaGuérin Méneville, and B. vanderhammeni Blöte) are redescribed and five species B.colossea, B.maai, B.rossi, B.versicolor, andB.wauensis)aredescribedasnew. Aneotypeis designatedfor B. exhausta and the maleofB. bicolorata is describedfor the first time. The synonymy ofB. exhaustato Cicadahastipennis Walker and Dundubiaparabola Walker is discussed and CicadaparallelaWalker is added as anew synonym tothis species. Akey to the males is provided. The monophyly ofthe exhausta group and its phylogenetic positionwithin the genus Baeturia are dis- cussed. The exhausta group is distributedinTimor, thegreaterpartofMaluku, and inNewGuinea withexception ofits central mountainranges. Mapsofdistributionarepresented. INTRODUCTION ofendemismformonophyletic groupsofcicadas (Duffels, 1986; Duffels & De Boer, 1990), indi- Thecicada genusBaeturiaStal, 1866, belongs to cating thatthese groupsevolvedwhen the areas a supposedly monophyletic group of New were still isolated fromAustralian New Guinea. Guinean and Australian genera definedearlier The phylogenetic relationships between these as the "Baeturia and related genera complex" monophyletic groups can hopefully tell us some- (De Boer, 1990). The distributionsof the New thing about the historical relationships between Guinean representatives of that complex reflect thevarious fragments of theislandarc, and may thegeological history of the island. New Guinea reflect the order inwhich they collidedwith the was formed after a series of collisionsof frag- Australian continent.For thesereasons, a phylo- ments ofan oceanic island arc, with the north- genetic and biogeographic revision of the ern craton of the Australian continent (Hamil- “Baeturia andrelated generacomplex" is under- ton, 1979; Daly et al, 1991; Pigram & Davies, taken. 1987; Pigram & Panggabean, 1984). Many of This publication is part ofa series of papers, these fragments can still be recognized as areas dealing with thephylogeny and biogeography of 127 monophyletic species groups, thatare recognized NCSU North CarolinaState University Insect Col- within the genusBaeturia, the largest genusof the lection,Raleigh complex. Revisions have already been published NhMW NaturhistorischesMuseum,Wien for the nasuta group (De Boer, 1982), the conviva RMNH NationaalNatuurhistorisch Museum (former- group(De Boer, 1986),the bloeteigroup(De Boer, ly: Rijksmuseum van Natuurlijke Historie), Leiden 1989), the viridis group(De Boer, 1992a), and the SMD StaatlichesMuseumfurTierkunde,Dresden guttulinervis group (De Boer, 1994b), the loriae SMN Staatliches MuseumfurNaturkunde, Stutt- group(De Boer, 1994a). gart The exhausta groupcontains 8 species, sharing TMB Termeszettudomany Muzeum,Budapest supposed synapomorphies in clasper-shape and ZMA Institute for Systematics and Population Bio- in shape of the laterallobes of the pygofer. The logy (Zoologisch Museum),Amsterdam species can also be separated from other species ZMB Institutfttr Spezielle Zoologie undZoologi- ofBaeturia by a slightly more slender and more sches Museum der Humboldt-Universitat, erect caudodorsal beak on the pygofer. The Berlin groupis distributed over New Guineaandsouth- ern Maluku, including the island of Timor, but seems concentrated in northwestern New The following geographical sources have been Guinea, where 3 ofits 8species occur. used: "The Times Atlas of the World (Compre- hensiveEdition)" (1968) anda "Listof NewGui- nea localities" published by the Bishop Museum MATERIALANDMETHODS (1966). Some of the terms used in the descrip- tions are explained inFigs. 8, 11, and 14. The material examined for this study is deposit- After overnight softening, male genitalia were edin thefollowing collections: examinedby pulling out thepygofer with asharp needleinserted betweenpygofer and8th abdom- AMNH American Museum ofNatural History, New inalsegment. Theaedeagus was pulled out atthe York same time, by inserting the needle between the AMS Australian Museum, Sydney claspers. For all specimens body length and BMNH Natural History Museum [formerly: British tegmen length were measured; other measure- Museum(NaturalHistory)], London ments are based on a maximum of ten speci- BPBM BerniceP.Bishop Museum,Honolulu mens. CAS CaliforniaAcademy ofSciences, Department ofEntomology, SanFrancisco CZL Instituto de investiga$ao cientificatropical Centro de Zoologia, Lisboa PHYLOGENY IPS InstituteofPlantSciences, Burnley, Australia IZW Polska AkademiaNauk, Instytut Zoologii, Themonophyly ofthe exhausta group Warszawa KBIN Koninklijk Belgisch Instituutvoor Natuur- Themonophyly of the exhausta groupisbased on wetenschappen, Brussel two apomorphies in male genitalia, but a third MAKB Museum"AlexanderKonig", Bonn genital charactermightalso be apomorphous. MSNG Museo Civico diStoriaNaturale"G. Doria", The species of the exhausta grouphave a short, Genova posteriorly directedclasper, with a distinct rec- MNP MuseumNationaled'HistoireNaturelle,Paris tangular clasper heel and a short and broadly Moul PersonalcollectionMr.M.S.Moulds,Sidney roundedventral hollow in its slightly downwards MVM MuseumofVictoria, Melbourne MZB MuseumZoologicum Bogoriense,Bogor curved apical part. The clasper is further con- MZS Musee Zoologique de1' Universiteet de la spicuous by the distinctly outcurving crest along Ville, Strasbourg its straight dorsal margin. As a result of this out- NBM NaturhistorischesMuseum, Basel curvation, both claspers together form a cup- 128 Fig. 1a.Phylogenetic relationships ofthe exhausta group. Numbersrefertoapomorphouscharacters discussed inthe tekst. Fig. 1b.Tentativecladogram oftheexhausta group. Numbersrefer toapomorphous charactersdiscussedinthe tekst. 129 shaped hollow aroundthe aedeagus (Fig. 6). This forBaeturiais hampered by the questionable phy- latter character is regarded apomorphous (8 in logenetic position of the loriae group (De Boer, Fig. la, 1 in Fig. lb). The claspers ofB. brongers- 1994a). The species of thatgroupcombinechar- mai Blote,l960, B. lorentzi De Boer, 1992, and B. acters that seem to be apomorphous for either viridis Blote, 1960, ofthe viridis group and those Baeturia or Gymnotympana. Current phylogenetic of the species ofthe exhausta groupshare a simi- analysis involving all species of the "Baeturia and larly shaped clasper hollow, but the claspers in related genera complex" shows that the loriae the viridis groupare broaderin lateral view, have group included in Baeturia is the most parsimo- aless strongly outcurving dorsalcrest and a con- nious solution(Fig. la). (Full results ofthis analy- vex dorsal margin (De Boer, 1992). In other sis willbe published later). species ofBaeturiathedorsal crestofthe clasper is The monophyly ofBaeturia, including the loriae generally less distinct, or absent, and the clasper group is based on the following apomorphies: 1) hollowgenerally longer, often more directedpos- a strongly curved aedeagus, tapering to its apex; teriad (compare the nasuta group De Boer, 1982; 2) lobate lateralcrests on the aedeagus; 3) ashort the conviva groupDe Boer, 1986; the bloeteigroup dorsal elongation of the aedeagal apex; 4) an De Boer, 1989; the viridis groupDe Boer, 1992a). oval aedeagal pore; and 5) a broad, triangle- Related genera have an altogether different shaped, medial thorn on the fore femur(3-7 in clasper. Fig. la). The lateral lobes of the male pygofer are Baeturia is, in this most parsimonious recon- strongly curved, quite often sharply folded mesi- struction, the sister group of Scottotympana De ad (Fig. 5), so that the distal margin ofthe Boer. The dorsal parts of the claspers in these pygofer is generally not visible in lateral view, generaare not fused to a collar around the base which is regarded apomorphous (9 in Fig la, 2 of anal valves, which is regarded apomorphous in Fig. lb). The pygofer lobes are only weakly (2 in Fig. 1a). Gymnotympana with itspresumed sis- curved mesiad in other Baeturia species. Similarly ter group Venustria Goding & Froggatt form the strongly curved pygofer lobes only occur in sister groupof Baeturia and Scottotympana, based species of the genus Gymnotympana Stal, which on large male opercula, extending medially of mustbeexplained as aparallelism. the meracanthus(1 in Fig. la). Furthermore, males of the exhausta groupcan The phylogenetic relationships of the exhausta be recognized by a very slender and erect cau- group with other species groups of Baeturia are dodorsalbeak (cf. Figs. 8-9). Thebeak is general- uncertain, as is expressed by the polytomy ofFig. ly more slender and more erect than in other la. The nasuta, conviva, guttulinervis, and viridis Baeturiaspecies, and longer thanwhat is common groups form amonophyletic groupbased on the in related genera. However, though this charac- synapomorphous posteriorly directed protuber- ter strongly varies between the various species ance on thelateral lobesofthe pygofer (10 inFig groups, a stout and more strongly bent cau- la). The nasuta and viridis groups are sister dodorsal beak might be synapomorphous for all groups, based on an angularly bent caudodorsal other Baeturia species together. In that case, it beak (13 in Fig. la) (De Boer, 1992a), and the could be argued, that a shape of caudodorsal conviva and guttulinervis groups are sister groups, beak, as found in the exhausta group, is the ple- basedon presumedsynapomorphies in theshape siomorphous statefor Baeturia. A decision on this ofthe pygofer andclaspers (11 and 12 inFig. la) problem depends on the position ofthe loriae (De Boer, 1992a, unpublished data). groupwithin the cladogram ofBaeturia (see the Current phylogenetic analysis indicates that next section). the loriaegroupat the baseof the cladogram and the exhausta groupas sister groupofthe remain- Thephylogenetic position ofthe exhausta group ing groups is the most parsimonious solution, but neithertheherein construedmonophyletic group Theconstruction of an unambiguous cladogram consisting ofthe nasuta, conviva, guttulinervis, viridis, 130 and bloetei groups, nor the groupformed by that lesser extent those ofB. vanderhammeni, are dense- group together with the exhausta group are ly set with long and thinsetae, on upper- and definedby clear apomorphic characters, they are underside. All other species ofthe exhausta group merely based on the most parsimonious way to have only very few setae on the veins.The tegmi- dealwithseveral homoplasious characters. na of these three species also tend to be longer, relative to body length, than in the other species of the exhausta group (see descriptions). This lat- Infragrouprelationships ter character might be synapomorphous for thesespecies (4 inFig. lb). The distributionof characters that are presum- Comparably densely "hairy" veinsare foundin ably significant for the phylogeny of the exhausta the nasuta group, the conviva group, and the gut- groupare discussed below. When appropriate, tulinervis group, in some species of the loriae the occurrence of these characters outside the group, and in some species of the viridis group, group is discussed. These data are summarised though the density of setae is variable between ina preliminary cladogram of the exhausta group the species ofthesegroups. The veinsare practi- (Fig. lb). cally bald inall other generaofthe "Baeturia and related generacomplex", but similar setae occur Shape ofpostclypeus in several species groups of the Prasiini, which B. rossi, B. versicolor, and B. wauensis have a dis- probably formthesister groupof thecomplex. tinctly swollen postclypeus (Figs. 54, 61) with a B. versicolor and B. wauensishave 9or 10 apical convex anterior margin (lateral view). The post- areas in thetegmina as presumed synapomorphy clypeus is weakly swollen in B. vanderhammeni, (6 in Fig. lb), all other species have 8 apical while in B. exhausta swollen and unswollen post- areas. Eight apical areas is common in Baeturia clypei occur. The postclypeus is not swollen, and and in most ofthe related genera. In several has an almost straight anterior margin (lateral species of Thaumastopsaltria (De Boer, 1992b) and view), inthe remaining species of thegroup. Gymnotympana (unpublished), the number of api- An unswollenpostclypeus is foundinall species cal areas varies between 10 and 15, and often of the bloetei group(De Boer, 1989) and several differs within individuals. The only male known species of the viridis group (De Boer, 1992a). A of B. karkarensis, belonging to the viridis group, swollen postclypeus is very common among also has 9 apical areas in the tegmen, but a otherspecies ofBaeturia. The phylogenetic signif- female that possibly belongs to that species has 8 icanceofthis characteris not clear. (De Boer, 1992a). Forefemur Shape of opercula B. maai, B. vanderhammeni, B. versicolor, and B. The distalpart ofthemale operculum ofB. maai, wauensis presumably form a monophyletic group. B. vanderhammeni, B. versicolor, and B. wauensis These species share a fairly short proximal spine tends to be more slender than in the remaining on the fore femur; shorter than the distance to species of the exhausta group,but such differences the middle spine (3 in Fig. lb). The proximal occur also in other groupsofBaeturia. MalesofB. spine on the fore femur is about as long as the versicolor and B. wauensis share a very short oper- distance to the middle spine in other species of culum, not reaching beyond the apex ofthe mer- the group. Similarly short spines also occur in acanthus(Figs. 59, 69). Suchshort maleopercula somespecies ofthe B. viridis group,however. regularly occur in the more distantly related con- viva, guttulinervis, nasuta, and viridis groups, but can Tegmina andwings presumably be regarded apomorphous for these Setae are formed on the veins of tegmina and two species (7 in Fig. lb), while the occurrence of wings of many species of Baeturia. The veins of short opercula inothergroupsmustbeexplained especially B. versicolor and B. wauensis, and to a by parallelism. 131 Fig. 2a.LocalitiesofBaeturiabicolorata, B.colossea, andB.exhausta. Fig. 2b.Localities ofBaeturiamaai,B. rossi,B.vanderhammeni,B.versicolorand B.wauensis. 132 Shape ofmalecaudodorsalbeak Larat, Laut, Teun, and Tomea) (De Boer, 1989). B. vanderhammeniand B. wauensisshare a very slen- Though B. macgillavryi is not recorded from New dercaudodorsal beak; the beak is oblong in dor- Guinea, it was remarked, that two specimens sal view (Figs. 43, 53). This shape ofbeak is from southern NewGuinea, and attributedto B. unique, and the might be synapomorphous for bloetei (the sister species ofB. macgillavryi) take a these two species and B. versicolor (5 in Fig. lb), somewhat intermediate position between these the more strongly curved beak of the latter two species (De Boer, 1989). H. ciliaris too, is species being an autapomorphy. widely distributed over the Moluccan islandsand also recorded from Timor, but the species Shape ofclasper extends northward into Mindanaoand the Palau B. exhausta and most specimens of B. bicolorata group of the Caroline Islands (Duffels, 1991). It share an almost straight distal margin ofthe is remarkable, that those species reaching the clasper (Figs. 1 1, 26-32), which is possibly southern Moluccasand Timor tendto havesuch synapomorphous for these two species (9 in Fig. wide distributions, while species restricted to the lb). The distalmargin of the clasper is concave, northernMoluccas are much more endemic(see marking the end ofthe dorsal crest, in all other the Diceropyga obtecta group,Duffels, 1977 andthe species ofthe exhausta group (Fig. 34 arrow) and BaeturiaconvivagroupDeBoer, 1986). in other Baeturia species that have a distinct dor- Itis supposed thatthe distributionsofB.exhaus- sal crest. The distal margin ofthe clasper is only taand B. macgillavryi are the result ofarecent dis- very weakly concave in B. maai, however. The persal from New Guinea, since many of the distal end of the dorsal crest of clasper is very south Moluccan islands only recently, less than 1 prominent and often somewhat protruding in B. millionyears ago, emerged (Fortuin & De Smet, versicolor and B. wauensis, this characteris presum- 1991). H. ciliaris has no near relatives on New ably synapomorphous for these two species (8 in Guinea, and has presumably a different biogeo- Fig. lb). graphical history. B. bicolorata, the sister species ofB. exhaustaand very similar to that species, is widely distributed BIOGEOGRAPHY in New Guinea, from Cendrawasih to the Papuan peninsula, occurring north, as well as Theexhausta groupis distributed in New Guinea south ofthe central mountainranges, and is fur- and Maluku, including Timor island. Two ther recorded from the Aru Islands. Only two species, B. bicolorata and B. exhausta, have a rela- cicada species are known to haveasimilarly wide tivelywide distribution. distribution inNew Guineaoccurring north and B. exhausta isrecorded from Timor, most ofthe south of the central mountain ranges: southern Moluccan and Banda islands, the Aru Guineapsaltria flava (Goding & Froggatt, 1904), islands, and the Mimika coast of New Guinea. which is also found in the eastern parts of the Though B. exhausta has not been recorded from Cape York Peninsula of Queensland (De Boer the north Moluccan islands of Halmahera, 1993a), and Aedeastria latifrons (Blote, 1960), Morotai or Talaud, the species is recorded from which has a slightly more limited distribution Bacan and Ternate (Fig. 2a). A similarly wide and does not occur on Cendrawasih or in east- distributionwas found forB. macgillavryi De Boer, ern Papua New Guinea (De Boer, 1993b). It is 1989, of the bloetei group, and for Hamza ciliaris curious to notice that boththese species are also (Linnaeus, 1758). The distribution area of B. recorded from theAruislands. macgillavryi includes Halmahera, Morotai and The remaining species of the exhausta group Talaud, but the species is not recorded from the have a much more limited distribution. Three eastern islands (Aru, Kai, and Tanimbar), andis species (B. maai, B. rossi, and B. vanderhammeni) not known from Sula and most of the smaller occur in the northernparts of New Guinea, B. Moluccan islands (Bacan, Damar, Haruku, maaiand B. rossi are endemic there, while B. van- 133 derh.amm.eniis alsorecorded fromBalimoinsouth- thorax (1.1-1.4 x in B. wauensis), of females 1.0- eastern Papua New Guineaand fromBisianumu 1.3 x. Tegmina of males 1.0-1.2 x as long as on the Papuan peninsula. Two species (B. colossea body length (1.2-1.4 x in B. wauensis), of females and B. wauensis) are endemic to northeastern 1.2-1.6x. Papua New Guinea, in and near the Finisterre Head:Postclypeus distinctly protruding beyond range,and onespecies (B. versicolor) is endemic to vertex lobes(Fig. 3), broadly roundedatanterior the Papuan peninsula (Figs. 2a-b). margin and 1.4-2.9 x as broad as long. Lateral By far mostofthe New Guineancicadas occur parts ofpostclypeus with 6-7 rows ofshort paral- in northern New Guinea or on the Papuan lel ridges, forming a broad band along lorum peninsula. The distribution of species or mono- (Fig. 4). Distance between eyes 1.4-2.0 x as long phyletic species groups in these areas is a com- as postclypeus width. Headonly slighdy narrow- mon pattern, found in Baeturia (De Boer, 1982; er thananteriorwidth ofpronotum, 2.2-3.1 x as 1989; 1992), Diceropyga (Duffels, 1977), Guineap- wide as long and 2.1-2.6 x as wide as distance saltria (De Boer, 1993a), Gymnotympana (unpub- betweeneyes.Pronotal collar 1.2-1.5x as wide as lished), and Thaumastopsaltria (De Boer, 1992b). head. Ocelli fairly large and close together. However, Diceropyga, Gymnotympana, and Thau- Distance between lateral ocelli 0.8-1.7 x as long mastopsaltria appear to be concentrated in the as distance between eye and lateral ocellus and Papuan peninsula, as far as their New Guinean 1.0-2.0 x the width of frontal ocellus. Distance species are concerned, while Baeturia seems to betweeneyes inmales 1.2-1.6x as wideas eye, in have most species in northernand western New females 1.3-1.8x. Guinea. Thorax: Pronotum with two pairs of deep oblique fissures; speckling generally most dense between these fissures and in medial band, but TAXONOMY medial bandoccasionally immaculate andlighter coloured. Pronotum 2.1-2.6 x as broad as long. Description oftheexhausta group Mesonotum greyish brown, or greenish, general- ly densely speckled, but less so in two semicircu- The species of the exhausta group showa striking lar spots at pronotal margin and inlateral bands, similarity, especially in malegenital structures. In and generally without black spots infrontof cru- other species groups the clasper shape alone ciform elevation. Mesonotum 0.7-0.8 x as long often suffices for identification on species level, as widthof pronotal collar. but in the exhausta group other characters (viz. Tegmina and wings: Hyaline. Tegmen with 8, postclypeus shape, operculum shape, numbers of in two species 9-10, apical areas, a very narrow apical areas in tegmina and numbers of tymbal subcostal area anda fairly narrow hyaline border ridges) are neededto distinguish the species. along its hind margin. Wing with 6 apical areas Body ofmales reddish brown, generally dense- and a slightly broader hyaline border along its lybrown speckled on head, thorax and predomi- hind margin. Venation ochraceous or greenish, nantly the dorsal parts of abdomen, sometimes in somespecies densely set withsetae. with a light and unspeckled middorsal band over Legs: Fore femur(Fig. 7) with row of three or whole length of body. B. versicolor unspeckled, four pointed spines, diminishing in length with a conspicuous pattern of olive greenand towards tibia. Most proximal spine shorter than, black markings. Females generally darker brown or aboutas long as,distance to middle spine. and more densely speckled than males, but Opercula: Consisting of a vaultedbasal part, females of B. bicolorata unspeckled, with greenish with a distinct crestaround its rectangular disto- head and thorax, and ochraceous abdomen. lateral corner, and a flat distal part. Male oper- Females often shorter than males, but with more culum with oval to oblong-shaped distal part, robust head and thorax, and longer tegmina. which generally covers the greaterpartoftymbal Male abdomen 1.3-1.8 x as long as headand cavity, and always reaches medially of meracan- 134 thus. Distal part of female operculum much KEY TO THE MALES smallerthan thatofmaleandsickle-shaped. Tymbal organ: Generally with seven parallel 1 a Veins oftegmina and wings densely set with short sclerotized ridges spanning the tymbal, while an setae 2 b Veins oftegmina and wingswith very fewsetae,prac- 8th, most proximal ridge, is only partly devel- tically bald 4 oped andreachesto abouthalfthetymbal width. 2 a Postclypeus weakly swollen ventrally. Tegmen with 8 B. rossi has six ridges and a half, B. maai has five apical areas.Operculum long, reaching beyond apex ridges and a half. ofmeracanthus B.vanderhammeni Abdomen: Male abdomen very delicate, dis- b Postclypeus distinctly swollen ventrally. Tegmenwith tinctly inflated, generally with reddish segmental 9or 10apicalareas.Operculum short, notreachingto apexofmeracanthus(Figs. 59, 69) 3 hind margins, andoften with ventrolateralrow of 3a Body greyish brown and densely brown speckled, dark spots on segments 3-7 or8. First tergite fair- especially on head, thorax and dorsal part of ly long, partly hidden under metanotum in B. abdomen, but without distinctcolour pattern. Caudo- versicolor and B. wauensis. Anteriormargin of2nd dorsal beak oblongin dorsalview,withalmostparallel tergite convex medially, but weakly concave in B. lateral margins(Fig. 53) B.wauensis b Bodylight ochraceous and unspeckled. Pronotumwith versicolor and B. wauensis. Lateralparts of 2ndter- olive green middorsalbandand collar.Cruciformele- gite weakly inflated and adjacent to tymbal. vation ofmesonotum and metanotum olive green. Ventral partof2ndtergite straight betweenaudi- Distinct black streak onfore femur and black spots at tory capsule and 1st sternite. Auditory capsules bases oftegmenandwing. Caudodorsalbeak triangu- distinctly swollen. First sternite broad and blunt- lar in dorsal view, its lateral margins convergingto ly rounded distally, forming a small distal lobe. apex (Figs.63,64) B.versicolor 4a Clasper with distinct bend in distal margin, marking Female abdomenmore solid than that of male. the endofdorsal crest (Fig. 34arrow) 5 Female caudodorsal beak sharply pointed at b Clasper with straightdistalmargin(Fig. 11) 7 apex. Ovipositor sheaths reaching to, or just 5 a Postclypeus distinctly swollen ventrally. Operculum beyond, apexofbeak (Fig. 21). verybroad,square-shaped (Fig. 86), completelycover- Male genitalia: Pygofer with very slender and ingtymbalcavity in ventralview and distinctly visible in dorsalview B.rossi erect caudodorsalbeak. Dorsal margin ofpygo- b Postclypeus weakly ornotswollen ventrally. Opercu- fer slightly convex, generally continuouly curved lum oblong(Figs. 38, 74), partly covering tymbal cavi- with rounding of beak. Lateral lobes ofpygofer ty in ventral view and hardly or not visible in dorsal strongly curvedor folded mesiad, forming blunt- view 6 ly roundedprotuberances at their distal corners. 6a Bodylength 24.1-27.4 mm. Frontalocellus aswide as, Ventral margin of pygofer angularly bent. orwiderthan, distance between lateral ocelli. Tymbal with8sclerotized ridges B.colossea Ventral margins converging to sharp angle at b Body length 21.5-21.6 mm. Distance betweenlateral base of pygofer (Figs. 5, 45, 73). Caudodorsal ocelli 1.2-2.0xaswideas frontalocellus. Tymbalwith beakpointed at apex. Claspers parallel, not fused 6sclerotized ridges B.maai at base, and thus not forming a ring-shaped col- 7 a Body lengh generally under 22 mm. Opercula not lar aroundbase ofanal valves. Clasper with dis- stronglydirected mesiad,clearlyvisible in dorsalview. Clasper straight directed posteriad, its dorsalmargin tinctly outcurving crest along its straight dorsal parallel with dorsal margin of clasper base (Fig. 11) margin. As a result of this outcurvation, both (Timor, Maluku,Banda, Key and Aru Islands, Mimi- claspers together form a cup-shaped hollow kacoast ofNew Guinea) B.exhausta aroundthe aedeagus (Fig. 6). Clasper baseform- b Body lengh generally over 22 mm. Opercula strongly ing a rectangular corner, here termed "clasper directedmesiad, not visible in dorsal view. Clasper oftenslightly upcurved, its dorsalmarginoften direct- heel". Apical part ofclasper fairly short and ed upwards relative to dorsal marginofclasper base strongly bent downwards, with sharply edged (Figs. 26-32) (New Guinea, Aru Islands) ventral incurvation or "clasper hollow." Bj .bicolorata Aedeagus S-curved, with two small laterallobes at basal curve. 135 DESCRIPTION OF THE SPECIES 1$, ZMA; Amboina,Doherty, Id, BMNH;Amboina, Suykerbuyk, Id1 det. Baeturia quadrifida Walk., 2d, 19, Baeturiaexhausta (Guérin Méneville, 1831) KBIN;Amboina, 1873,O. Beccari, Id, 19, BMNH;same data Id1 det. B. quadrifida, 3d, 29, MSNG; same data but (Figs. 1-17) xii.1874, Id1det. B. quadrifida, Id1, MSNG; Hitu,Amboina, xii.1891, Exp. Martin, 19, BMNH; Leitimor, Amboina, Cicada exhausta Guerin Meneville, 1831: PI. 10 Fig. 6; xii.1891, Exp. Martin, lcf, BMNH; Ambon, 1880, Ten Guerin Meneville, 1838: 181; Walker, 1850: 120; Walker, Cloet, 2cf, 19, ZMA; Ambon,v-viii.1865, Hoedt, Id1, 29, 1858:30; Walker, 1868: 92. RMNH; Ambon, coll. Dr D. Macgillavry, 19, ZMA; Baeturiaexhausta; Distant, 1888: 487; Distant, 1892b:xiv, Ambon,Forsten, Id, RMNH; Ambon,Ludeking, 19, 149,PI. XVFigs. 13, 13a-b;Breddin, 1900: 181; Horvath, RMNH; Waai, various dates between 14.V.1960 and 1900: 642; Kirkaldy, 1905: 330 (partim: Maluku); Distant, 15.viii.1967,A.M.R.Wegener, 29d, 89, BPBM;samedata 1906: 157; Kirkaldy, 1907: 308 (partim: Maluku); Distant, 22.V.1960, Id"; 31.V.1960, 19; 2.vi.l960, Id; 28.vi.1960, 1911: 389; Distant, 1912:599; Kirkaldy, 1913: 8; Distant, 19; 20.iv.1963, Id, all ZMA; same data 3.iii.1966, Id, 1914: 346; Schmidt, 1926:222, 257; Myers, 1928:55, 60, 24-.iii, 1966, 2d, CAS; BACAN: Batjan Waigaua, Figs. 5-9, 11-17, 19-21 (partim: Maluku); Schmidt, 1928: 14.vii.1953, Id, MZB;BURU: Bouru, Id,BMNH;Bouro, 110;LaUemand, 1931: 78; Kato, 1932: 184, PIXXVIFig. Higgins 69, coll. CamillevanVoixem, 19det.Baeturiaparal- 9;Blote, 1958: 265; Metcalf, 1963: 248-249; DufTels, 1977: lela Walk. / Baeturia convivaStal, KBIN; Bouru, Doherty, 209; DufTels & Van der Laan, 1985: 252; De Boer, 1986: Distant coll. 1911-383,3d, BMNH; Bara, W. Bouru, 173;DeBoer, 1989: 1,2,3, 40;DeBoer, 1992a: 166. ix.1899, 2dBaeturiaexhaustaGuer. det.J.G. Myers,2d, 19, Cicadahastipennis Walker, 1858: 30;Stal, 1862:483. BMNH; Buru Station 1, 1921,leg L.J. Toxopeus, Id neo- Baeturiahastipennis; Blote, 1958:265,267,Fig. 1 type, Id, RMNH; same data but Station 9, 19,RMNH; Cicadaparallela n. syn.Walker, 1868: 94. DAMAR: Insel Dammer, Ost Indien, W.H. Muche Cicadaparallela in synonymy of Baeturiaconviva; Distant, Radeberg ankauf, Id, SMD; HARUKU: Haroekoe, 1892b:xiv, 148, 149, PI XIV Figs. 25, 25a-b; Horvath, SnelliusExp., 3-7.V.1930, 4d, RMNH; Haroekoe, ii.1892, 1900: 642;Distant, 1906: 156;Metcalf, 1963: 247. Oeliasers, Exp. Martin, Id, BMNH; NOESSA LAOET: Baeturiaparallela; Blote, 1958: 266;Duffels& Van der Laan, Noessa Laoet, Ludeking, Id Baeturiaparabola Walk, det 1985: 254. H.C. Blote, Id det. Baeturiaexhausta Guer.,39, RMNH; Dundubiaparabola Walker. 1858: 6. LARAT:Larat,F. Muir, 4d, 19, BPBM;SERAM: Cer. E., Baeturiaparabola; Blote, 1958: 265,267, Fig. 2; Duffels & Idtype Cicadaparallela Walker,BMNH; Ceram, IdCicada VanderLaan, 1985: 254. exhausta,MVM;Ceram, Id, 19, AMS;Ceram, Id, 19type Cicada hastipennis Walker, 19det. exhausta Guerin / conviva In the following literature B. exhausta is men- Still, BMNH; Ceram, Forsten, 29, RMNH; Ceram, 1913- tioned, but, according to the geographical data, 504, E. Stresemann, Idtype Pomponiajacobsoni Chinadet. W.E. China, 19, BMNH; Ceram I., ix.1909, W. Stalker, some other species is meant: Dohrn, 1859: 74 Id, BMNH; Seram, Id, SMD;G. Binaia, N. slope, site 9, (Malakka); Myers, 1929: 162, 166, 171 (Samoa); 1000 m, viii-ix. 1987,J.D. Holloway, Id, BMNH; G. Lallemand, 1935: 677 (Solomon Islands); Kobipoti,N.side,site4,250m,viii-ix.1987,J.D.Holloway, Lallemand& Synave, 1953:233 (Sumba). Id, BMNH; same databut site 5, 550 m, Id; summit ridge,site 7, 1370 m, 3d, 29, all BMNH; ManuselaWae Materialexamined: IRIAN JAYA: NEW GUINEA (W): MualPlain, ix.1987, M.J.D. Brendell, Id, 29, BMNH; MimikaR., vii.1910, A.F.R. Wollaston, lcf Baeturiavander- Piroe, i. 1909,F. Muir, 2d, 19, BPBM; same data but hammeniBlote det. H.C. Blote, 19,RMNII; ARU: Aru, ii.1909, 19, BPBM; Solea, site 2, 50 m, viii-ix.1987,J.D. 1909, W. Stalker 1910-127, lcf, BMNH; KAI: lies Key, Holloway, 4d, 29, BMNH; SULA BESI:Sula, Wallace, coll.Noualhier, lcf, 1$, MNP;Ins.Key, 2$,TMB;Kei, lcf Id Cicada exhausta, MVM; Sula Besi, Doherty Ex. coll. Cicada exhausta, MVM; Key eil. Dangka Exb., 22.iv.1922, Fruhstorfer, Id, 19, TMB;Taliaboe, Zs Archipel, iv.1905, Papakula, 1cT, MZB; Key Ins, Planten, 2cf, 39, BMNH; v.Nauhuis, Id, RMNH; TANIMBAR: Insula Tanimbar, Key Inseln,Tual Langgur, 2cf, NhMW; Toeal, 39, MZS; Coll. Dr D. Macgillavry, 3d Tibicenpucillusdet.M.Gill, 29, Key, 29, IZW; Gn Daab, 1922,H.C. Siebers, ld\ IZW; ZMA;TERNATE: Ternate Malay Archipelago, 1903-31, Toeal, 1922, H.C. Siebers, 19, IZW; MYSOOL: Misool W. Doherty, 3d, 29, BMNH; same data Id, ZMA; Id. (W), 0-75 m, 8.ix-20.x.l948, M.A. Lieftinck, lcf, MZB; TEUN: Teoon,Friese, Id, TMB;TOMEA: Tomia, 1903- MALUKU: Cer. or Amb., Obeifo [?], lcf type Dundubia 188, 19, BMNH; NUSSATENGGARA: TIMOR:Timor, parabola Walker, BMNH; AMBON: Amb, Wallace, 29, 19 det.parallela Walk., BMNH; Baikal, 200-300 m, 14- MVM;Amb,Amboina,Wallace, lcf Cicadaexhausta, MVM; 24.xii.1963,J. Sedlacek, 29, BPBM; Dili (near) [Ermera], , Amboina, ld\ BMNH; Amboina,A. Kolber, Id", 29, 1140 m,25.xi.1967, Raimundo, Id, CZL; samedata but KBIN; Amboina,F. Muir,9c?, 109, BPBM;samedata lcf, 975 m, 12.ix.1968, Id; samedatabut 1150 m, 10.xii.1968, 136
