The Evolving Role of Chemical Synthesis in Antibacterial Drug PDF
Preview The Evolving Role of Chemical Synthesis in Antibacterial Drug
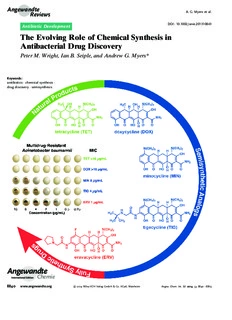
Angew.andte A.G. Myers etal. Reviews DOI:10.1002/anie.201310843 Antibiotic Development The Evolving Role of Chemical Synthesis in Antibacterial Drug Discovery Peter M. Wright, Ian B. Seiple, and Andrew G. Myers* Keywords: antibiotics·chemicalsynthesis· drugdiscovery·semisynthesis Angewandte Chemie 8840 www.angewandte.org (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim Angew.Chem.Int.Ed.2014,53,8840–8869 Angewandte Antibacterial DrugDiscovery Chemie T hediscoveryandimplementationofantibioticsintheearlytwentieth From the Contents century transformed human health andwellbeing. Chemical synthesis 1.Introduction 8841 enabled the development of the first antibacterial substances, orga- noarsenicalsandsulfadrugs,buttheseweresoonoutshonebyahostof 2.ChemicalSynthesis Ushers in more powerful and vastly more complex antibiotics from nature: theGolden Age ofAntibiotics penicillin,streptomycin,tetracycline,anderythromycin,amongothers. Discovery 8842 These primary defences are now significantly less effective as an 3.Semisynthesis: APowerful unavoidable consequence of rapid evolution of resistance within Postwar Enginefor pathogenic bacteria, made worse by widespread misuse of antibiotics. AntibacterialDiscovery 8845 For decades medicinal chemists replenished the arsenal of antibiotics by semisynthetic and to a lesser degree fully synthetic routes, but 4.FullySynthetic Antibacterials, economic factors have led to a subsidence of this effort, which places 1940-Present 8851 society on the precipice of a disaster. We believe that the strategic 5.ChemicalSynthesis asa Path application of modern chemical synthesis to antibacterial drug Forward 8857 discoverymustplayacriticalroleifacrisisofglobalproportionsisto be averted. mechanismsareemerging,[5]andinfec- tions caused by multidrug-resistant Gram-negative bacteria are becoming 1.Introduction particularlydifficulttotreat.Theproblemisexacerbatedby the ease of international travel and increasing global pop- The emergence of pathogenic bacteria resistant to many ulationdensities.Ourcurrentarsenalofantibioticsissteadily orallcurrentantibioticsisamajorpublichealthconcernand losing its efficacy and there is little sign that it will be one of particular importance in clinical settings. The World adequately replenished in the near future.[3,6] The develop- Economic Forum recently identified antibiotic resistance as ment of bacterial resistance is an inevitable consequence of oneofthegreatestthreatstohumanhealthinitsGlobalRisks evolution, and without continued replenishment of our 2013report.[1]TheCenterforDiseaseControlandPrevention arsenal of antibacterial agents, humanity runs the risk of released a summary of antibiotic resistance threats in the returningtoapre-antibioticera. UnitedStatesin2013,outliningthe“potentiallycatastrophic In this Review we examine the 100-year history of consequences of inaction.”[2] Natural selection, assisted by antibiotics discovery and development from its dawning globalmisuseofexistingantibiotics,andtheslowingpaceof with the synthesis of the first arsenical agent to those few discovery of new antibiotics conspire to place society at or antibiotic candidates that are currently in late-stage clinical nearacrisispoint.Theinnovationdeficitisinlargemeasure evaluation,[6] highlighting the essential and evolving role of due to the fact that many major pharmaceutical companies chemical synthesis throughout. Our objectives are to recog- have abandoned antibacterial research and development, nize select key contributions of the thousands of scientists atrendwhichhascreatedorattheveryleastcontributedto who have provided the modern antibacterial pharmacopeia the steep decline in the number of new antibacterials and to make the point that the clearest path forward to launched inthe last 30years(Figure1).[3] Meanwhile, resist- discover future generations of life-saving medicines will ance rates around the world are rising,[4] new resistance involvechemicalsynthesisasitscoreactivity. More specifically, we suggest that the development of practical, diversifiable, fully synthetic routes to antibiotic natural product scaffolds that are not yet accessible in this waypresentsthegreatestopportunityforrapiddiscoveryand developmentofnewantibioticsinthenearterm(5–20years). By this analysis, many of the natural product classes that emerged during and defined the golden era of antibiotics discovery (ca. 1940–1960) represent underutilized resources. As we argue in this Review, the development of practical, fullysyntheticroutestoantibacterialmoleculesisatried-and- tested strategy whose perceived constraints (molecular size andcomplexity,scalability)needtobereevaluatedinlightof [*] Dr.P.M.Wright,Dr.I.B.Seiple,Prof.Dr.A.G.Myers DepartmentofChemistryandChemicalBiology,HarvardUniversity Figure1. NumberofnewantibacterialsapprovedbytheFDAin5-year Cambridge,MA02138(USA) periodsfrom1983topresent.[3] E-mail:[email protected] Angew.Chem.Int.Ed.2014,53,8840–8869 (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim 8841 Angew.andte A.G. Myers etal. Reviews advances in modern chemical synthesis, both strategic and sectionof thisReview, weidentify specificopportunitiesfor methodological. We believe that ambitious, translational chemical innovation to fuel future antibiotic drug develop- chemical synthesis must be a core activity of antibiotics ment.Lastly,wehastentonotethatwhileourexpertiseand researchmovingforward,asithasbeensincetheinceptionof emphasisischemistry-centered,werecognizethatthefieldof thefield. antibiotic discovery would not exist nor could it advance withouttheessentialcontributionsofindividualsfrommany disciplines: isolation scientists, microbiologists, crystallogra- 1.1.ScopeandFocusofthisReview phers, clinicians, geneticists, toxicologists, and formulations experts,amongothers. The vast literature of antibiotics includes several fine reviewarticles,[7]manyofthempublishedinthisjournal.[8]For clearandcomprehensiveaccountsofallaspectsofthisfield— 1.2.NomenclatureofthisReview includingresistance,mechanismsofaction,microbialscreen- ingforantibioticnaturalproducts,antibioticbiosynthesis,and All antibiotics used in human therapy since the dawn of drugdevelopment—wedirectreaderstotwoexcellenttexts, theantibioticseraintheearly1900scanbedividedintothree oneauthoredbyChristopherWalsh[9]andtheothereditedby distinct categories according to how they were ultimately Thomas Dougherty and Michael Pucci.[10] A detailed under- manufacturedonlargescale.Thesecategorizationsfollow. standing of the molecular basis for antibiotic activity and Natural products: compounds manufactured directly by resistance is critical to the success of any drug development large-scalefermentationofbacteriaorfungi. program, but these factors are not the focus of this Review. Semisyntheticantibacterials:compoundsmanufacturedby Withtheexceptionofanoverviewofrifampicin,thecomplex, chemical synthesis using as starting material a natural prod- extraordinarilychallenging,andimportantproblemofdevel- uct. oping drugs to treat tuberculosis is also not covered here. Fully synthetic antibacterials: compounds that are manu- While others have previously articulated the importance of facturedbyfullysyntheticroutes. chemical synthesis in antibiotics drug discovery,[7f,8c,d] our Throughout this article we have attempted to adopt the focal point is the development of platform technologies to green,blue,andredcolor-coding,respectively,inFiguresand access natural product scaffolds (broadly defined) by con- Schemes to clarify the means of manufacture of the anti- vergent,component-based,fullysyntheticroutes.Ourinten- bioticspresented.Whilenewcategoriesmayyetemerge,such tionistoilluminatetheevolvingrolechemicalsynthesishas as antibiotics produced by metabolic engineering, here we playedinthediscoveryanddevelopmentofnewantibacterial focus on processes that have already yielded clinical agents. agents so as to make clear its potential to contribute to the alleviation of the current innovation deficit. In the final 2.Chemical Synthesis Ushers in the Golden Age of Antibiotics Discovery PeterM.Wrightwasbornandraisedin Southport,U.K.HestudiedchemistryatSt. John’sCollege,UniversityofOxford,under 2.1.DiscoveryandDevelopmentoftheFirstAntibiotics theguidanceofProf.GeorgeW.J.Fleet,and conductedresearchwithProf.TimothyJ. Thefirsteffectivetreatmentforabacterialinfectionarose Donohoe.HethenmovedtotheU.S.to fromaconvergenceofdisparateadvances,includinganearly pursuegraduatestudieswithProf. chemical synthesis of aniline, Paul Ehrlich(cid:2)s “magic bullet” AndrewG.MyersatHarvardUniversity, hypothesis, and the development of the first treatments for workingonthesynthesisoftetracyclineanti- Africansleepingsickness.In1854theFrenchchemistAntoine biotics.HeisnowanAssociateatMcKinsey &CompanyinNewYorkCity. B(cid:3)champachievedthefirsteconomicalsynthesisofanilineby reduction of nitrobenzene with iron in the presence of IanB.SeiplereceivedhisB.Sc.fromthe AndrewG.MyersreceivedhisB.Sc.fromthe UniversityofCaliforniaatBerkeleyunder MassachusettsInstituteofTechnologyin thetutelageofProf.DirkTrauner,andin 1981,andwentontopursuegraduate 2006joinedthelaboratoryofProf.PhilS. studieswithProf.EliasJ.CoreyatHarvard BaranattheScrippsResearchInstituteas University.Hestartedhisindependentcareer anNSFPredoctoralFellow.Duringhis attheCaliforniaInstituteofTechnologyin graduatestudieshedevelopedfullysynthetic 1987,andmovedtoHarvardUniversityin routestopyrrole-imidazolealkaloids,includ- 1998,whereheiscurrentlyAmory ingpalau’amine.HeiscurrentlyanNIH HoughtonProfessorofChemistry.His postdoctoralfellowinthelaboratoryofProf. researchprograminvolvesthesynthesisand AndrewG.MyersatHarvardUniversity. studyofcomplexmoleculesofimportancein biologyandhumanmedicine.Heandhis studentshavealsodevelopedmethodsof generalutilityintheconstructionofcomplex molecules. 8842 www.angewandte.org (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim Angew.Chem.Int.Ed.2014,53,8840–8869 Angewandte Antibacterial DrugDiscovery Chemie hydrochloricacid,adiscoverythatcatalyzedthegrowthofthe a simple composition and extraordinary reactivity, which synthetic dye industry.[11] Subsequent efforts to prepare permitted a wide variety of modifications.” According to aniline derivatives led B(cid:3)champ to synthesize a compound Ehrlich,atoxyl“enabledchemotherapytodistanceitselffrom knownasatoxylin1859bythereactionofanilinewitharsenic purely empirical trial and error testing and to introduce acid.ThechemicalstructureofatoxylproposedbyB(cid:3)champ chemicalsynthesis.”[17] waslaterrevised(seeScheme1). Bertheim, Ehrlich and co-workers proceeded to synthe- Inthelatterpartofthe19thcentury,PaulEhrlichbegan size hundreds of structurally related organoarsenic com- his prodigious search for a “magic bullet,” a molecule that pounds based on atoxyl and test them for activity against could combat disease-causing organisms.[12] Ehrlich was trypanosomes. Following a suggestion by Erich Hoffmann, broadly interested in fully synthetic dyes, their apparent theseorganoarseniccompoundswerealsotestedagainstthe selective affinities for living tissues, and their therapeutic microorganismfoundin1905byHoffmannandSchaudinnto potential. He hypothesized that the affinity of specific cell bethecausalagentofsyphilis.[18]Thisworkculminatedinthe types for dye molecules could be harnessed to selectively discovery of salvarsan (Figure2),[19] the first effective treat- destroymicroorganismsinthebodywithoutdamaginghuman ment for syphilis and the first antibacterial drug. Salvarsan cells.Anearlybreakthroughcamein1891whenEhrlichand wasalsoknownas“Compound606,”enumeratingitsplacein Paul Guttmann reported that two patients suffering from thesequenceofapproximately2000fullysyntheticmolecules malariahadbeensuccessfullytreatedwiththefullysynthetic evaluated during Ehrlich(cid:2)s investigations, and it rapidly thiazinedyemethyleneblue,[13]possiblythefirstexample of became the most widely prescribed drug in the world. By afullysyntheticdrugbeingusedinhumanmedicine.Ehrlich 1920, 2million doses of salvarsan and neosalvarsan (“Com- wasalsoactivelyinvolvedinthedevelopmentofsyntheticdye pound 914,” a more water-soluble derivative of salvarsan) therapeutics for African sleeping sickness, which ravaged werebeingproducedannuallyintheU.S.alone.[20]Salvarsan equatorial Africa around the turn of the 19th century in an was very difficult to administer and had terrible side effects epidemic that claimed between 300000 and 500000 lives.[14] (including deafness), and chemotherapy remained a highly HisinterestwaspiquedbyapaperbyH.W.Thomasin1905 controversialidea.[21] demonstrating that B(cid:3)champ(cid:2)s atoxyl exhibited activity Intheearly1900sHeinrichHçrlein,directorofpharma- against trypanosomes, including the causative organism of ceuticalresearchattheGermanchemicalconglomerateI.G. sleepingsickness.[15] Farben,initiatedamajorefforttofindchemotherapeuticsfor Working under Ehrlich(cid:2)s direction, Alfred Bertheim bacterialinfections.[22]Hçrleinandchemistcollaboratorshad determined in 1907 that the chemical structure of atoxyl previously discovered that addition of sulfonamide substitu- had been incorrectly assigned: atoxyl was p-aminophenyl ents to synthetic dyes often strengthened their binding to arsenic acid (containing both amine and arsenic acid func- wool and silk fibers. They reasoned that the search for tionalgroups,seeFigure2),notanarsenicacidanilide(aless achemotherapeuticagentcouldbuilduponthisinsightfrom easily derivatized structural isomer containing a nitrogen- dyechemistry,inthatastructuralmodificationthatenhanced arsenicbond),ashadbeensuggestedbyB(cid:3)champ.[16]Thiswas amolecule(cid:2)saffinityforfiberscouldalsoincreaseitsaffinity a momentous discovery, as noted later by Bertheim: “Prob- fortheprotoplasmofbacteria.[23] ablyforthefirsttime,abiologicallyeffectivesubstanceexisted Around 1927, I.G. Farben chemists Fritz Mietzsch and whosestructurewasnotonlyknownpreciselybutalsowasof Joseph Klarer began to synthesize azo dyes for biological Figure2. Earlyhistoryofantibioticsdiscoveryanddevelopment. Angew.Chem.Int.Ed.2014,53,8840–8869 (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim www.angewandte.org 8843 Angew.andte A.G. Myers etal. Reviews testing. Several factors led them to investigate azo dyes: functional group nor the dye character of prontosil were numerous azo compounds with promising activity against responsible for its therapeutic effect. During the ensuing trypanosomes had been discovered during earlier efforts to decade chemists synthesized more than 5000 structural find therapeutics for sleeping sickness;[14] the azo dye variants of sulfanilamide, and a number of them were chrysoidine had been found to exhibit in vitro bactericidal launched as drugs.[23] One of these, sulfapyridine (known effects in 1913;[23] I.G. Farben dominated the global market familiarly as “M&B” after the British manufacturer May & for synthetic dyes, so the expertise and facilities required to Baker),wasusedtotreatWinstonChurchillduringaboutof prepare azo dyes were available in-house; and azo com- pneumoniainthewinterof1943.[24]Somesulfadrugssuchas pounds of wide structural variability were chemically acces- sulfamethoxazole are still used today, but problematic side sible(akeyreasonthiscompoundclasswasappealing).[22]By effectsandthespreadofresistancedrovemanyantibacterials 1932,MietzschandKlarerhadsynthesizedmorethan300azo from this class out of favor.[25] It is noteworthy that in the compounds, including a series containing sulfonamide sub- historyofhumanmedicinethefirsttwoantibioticsclassesof stituents,andprovidedthesefortestingtoGerhardDomagk clinicalutilitywerenotnaturalproduct-based,butwerefully andotherswhohaddevelopedasuiteofinvitroandinvivo synthetic substances that arose from extensive chemical biological assays to determine utility against streptococcal synthesisandserendipity. infections(amongothers).[22] Domagk discovered that the red dye prontosil produced incredible curative effects in mice previously injected with 2.2.WorldWarIICatalyzesProductionofPenicillinby lethal doses of streptococci. In the years that followed Fermentation,butnotChemicalSynthesis[23] prontosil saved the lives of a 10-month-old baby suffering fromstaphylococcalsepticemiaand,famously,Domagk(cid:2)sown Oneofthekeyscientificbreakthroughsofthe20thcentury 6-year-olddaughter.In1935,thesameyearasthecommercial occurred when Alexander Fleming discovered in 1928 that launch of prontosil, it was revealed by researchers at the asubstanceproducedbythefungusPenicilliumchrysogenum PasteurInstituteinParisthattheactiveprincipleofthefirst (formerly known as Penicillium notatum) exhibited antibac- “sulfa drug” was the simpler substance known as sulfanila- terialactivity.[26]Althoughthisfindingwasmadepriortothe mide(Figure2,Scheme1),acompoundveryeasilyprepared key achievements of Domagk and collaborators, the fully inthelaboratoryevenbytherelativelyprimitivemethodsof synthetic sulfa drugs found widespread clinical use many the day. This work demonstrated that neither the azo yearsbeforepenicillinbecameavailableforthetreatmentof bacterialinfections.NearlyadecadepassedfollowingFlem- ing(cid:2)s famous discovery before Howard Florey and Ernst Chain received a grant from the Rockefeller Foundation to isolate penicillin and investigate its biological properties. In 1940, the Oxford team member Norman Heatley demon- strated that treatment with crude penicillin significantly extended the lives of mice previously injected with a lethal strain of Streptococcus.[27] The landmark 1940 report in The Lancet begins as follows: “In recent years interest in chemo- therapeuticeffectshasbeenalmostexclusivelyfocusedonthe sulphonamidesandtheirderivatives.Thereare,however,other possibilities,notablythoseconnectedwithnaturallyoccurring substances.” In February 1941, multiple doses of partially- purified penicillin broth were administered to an Oxford policeman suffering from a staphylococcal infection.[28] The policeman(cid:2)sconditionimproveddramaticallyfollowingtreat- mentwithpenicillin,butafterfivedaysthelimitedsupplyhad been exhausted and the policeman succumbed to the resurgent infection. Florey and Chain needed much larger quantities. By1941,Britishindustrywasengrossedinthewareffort andlackedtheresourcestotacklealarge-scaleexperimental project.UsinghisRockefellerconnections,Floreycrossedthe AtlanticandpetitionedAmericanpharmaceuticalcompanies to consider mass-production of their therapeutic compound by fermentation. His timing was propitious. In June 1941, President Roosevelt established the Office of Scientific Research and Development (OSRD), a federal agency responsible for coordinating scientific and medical research relating to national defense. The Allies urgently needed to Scheme1. Chemicalsynthesisofsalvarsanandprontosil. find new treatments for the vast number of troops with 8844 www.angewandte.org (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim Angew.Chem.Int.Ed.2014,53,8840–8869 Angewandte Antibacterial DrugDiscovery Chemie disease and wound infections. Sulfa drugs were a hugely carbodiimidereagents.[36]Thistransformationbecamethekey important medical breakthrough but also had significant step in the first fully synthetic route to a natural penicillin limitations—theirspectrumofactivitywasnarrow,andsome (penicillinV), published in 1957 by Sheehan and Henery- bacteriaacquiredresistancerapidly.Furthermore,production Logan(Scheme2).[37] of these antibiotics was concentrated in Nazi Germany. The CommitteeonMedicalResearchofOSRDinitiatedamassive projecttoproducepenicillin:onearmoftheprojectaimedto maximizeproductionofpenicillinbyfermentation,whilethe othersoughttodevelopafullysyntheticroute. This unprecedented convergence of governments, phar- maceuticalcompaniesandacademicscientistssparkedrapid scientific innovation. Regulatory barriers were knocked down—even impure penicillin had curative effects—and intellectual property concerns were temporarily cast aside.[29] Pfizer scientists James Currie and Jasper Cane achieved a landmark advance by the implementation of deep-tankfermentationtechniquesforpenicillinproduction, dramatically increasing the production of this life-saving drug.[30]Meanwhile,thesynthesiseffortinvolvingmorethan 1000 chemists and 39 major laboratories failed to produce aviablechemicalsynthesisofpenicillinandwasterminatedin 1945.[23] Disagreement over the true chemical structure of penicillinmeantthatdifferentgroupsweretryingtosynthe- sizedifferentmolecules.Ironically,ateamofchemistsledby Vincent du Vigneaud did manage to synthesize a minute quantity of penicillinG in spite of the fact that they were targetingastructurethatlaterprovedtobeincorrect.[31]All Scheme2. FullysyntheticapproachestopenicilinVand6-aminopeni- efforts to synthesize what turned out to be the correct cillanicacid.[23] structure of penicillin, containing a so-called b-lactam or 4- membered cyclic amide function, failed due to the dual challenges of strain and sensitivity posed by the critical b- InMarch1958,Sheehanreportedatasymposiumthathis lactam ring.[32] This “diabolical concatenation of reactive group had prepared a compound known as 6-aminopenicil- groups”[33] at the core of the penicillin molecule remained lanicacid(6-APA)bybothfullysyntheticandsemisynthetic essentiallyinaccessibleafterperhapsthelargestcoordinated routes, the first public disclosure of a compound that would project (albeit a fairly short-lived one) in the history of prove to be critically important to the future discovery of organicsynthesis. dozensofnewb-lactamantibiotics,allwith modificationsof theC6sidechain.[38]Thefollowingyear,scientistsatBeecham ResearchLaboratoriesintheU.K.reportedtheisolationof6- 2.3.AGlimmerofHopeforaPractical,FullySyntheticPathway APA from penicillin fermentation broths (having submitted toPenicillins a patent application in 1957),[39] and soon thereafter this intermediate, now produced by fermentation, became the The failure of the penicillin synthesis project during dominant precursor for production of semisynthetic penicil- WWII led Ernst Chain to declare in 1946 that the molecule lins.Duetothenumberofstepsinvolvedandthelowoverall would remain inaccessible by fully synthetic means “unless yield Sheehan(cid:2)s fully synthetic route to penicillin was not someone invents an entirely new technique unknown to competitivewithmanufacturebyfermentation-semisynthesis, chemistry.”[34] Shortly thereafter, despite a precipitous drop buthispioneeringsyntheticeffortshadledtothediscoveryof inresearchefforts(andresearchfunding)directedtowardthe 6-APA and thereby the preparation by semisynthesis of development of a fully synthetic route to penicillins, John structuralanalogsthatcouldnothavebeenpreparedbyother Sheehan began making progress toward his landmark labo- means. ratorysynthesisofpenicillinV.Hisresearchinthisareawas made possible by the long-term support of the Bristol company. In 1950, Sheehan reported the total synthesis of 3.Semisynthesis: A Powerful Postwar Engine for apenicillinderivativebearinganovel5-phenylsubstituent.[35] Antibacterial Discovery Althoughthisnon-natural,fullysyntheticanalogwasinactive, theworkmarkedanimportantstepforward.Assuggestedby Bacteria and fungi have continuously evolved over Chain, a fully synthetic route to penicillins only became approximately 109years, producing compounds that confer accessible following a transformative chemical innovation. anevolutionaryadvantagebykilling(other)bacteria,notby Sheehan brought forward this innovation by inventing an theirefficacyintreatinghumanswithopportunisticbacterial extremely mild method for formation of amide bonds using infections. The evolutionary pressures of human pharmaco- Angew.Chem.Int.Ed.2014,53,8840–8869 (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim www.angewandte.org 8845 Angew.andte A.G. Myers etal. Reviews kinetics, safety, oral bioavailability, and efficacy only came tetracycline (Terramycin).[44] Chlorotetracycline and oxyte- into play in the 1940s when medicinal chemists began to tracycline were found to be active against a wide range of modifyfermentationproductswiththeobjectiveofobtaining Gram-positive and Gram-negative bacteria—together with safer,moreefficacious(andproprietary)antibiotics,aprocess chloramphenicol(seeSection4.1),theywerethefirst“broad- we refer to as “human chemical evolution.” A primary spectrum” antibiotics. As a brief aside, Gram-positive and methodbywhichhumanshavediscoveredanddevelopednew Gram-negativebacteriaaresocalledbecauseoftheirdiffer- antibacterial therapies for more than 60years has been ent responses to a common staining protocol developed by semisynthesis: chemical synthesis using natural products as Hans Christian Gram. All bacterial cells are bounded by startingpoints. a cytoplasmic membrane, a lipid bilayer that tends to be permeabletouncharged,lipophilicmolecules.Gram-negative bacteria(suchasEscherichiacoli,Klebsiellapneumoniae,and 3.1.OriginsofAntibacterialSemisynthesis Pseudomonasaeruginosa)alsohaveanoutermembranethat is significantly less permeable to lipophilic molecules. In Semisynthesis came to the forefront of antibacterial addition, Gram-negative bacteria often possess multidrug discovery efforts following innovative chemical alterations efflux pumps that expel many antibiotics. As a result, the ofnaturallyoccurringaminoglycosidesandtetracyclines.The development of antibiotics with activity against Gram-neg- firstaminoglycosideantibioticwasdiscoveredin1943,when ativebacteriaisparticularlychallengingandcurrentlyrepre- Albert Schatz, a graduate student working with Selman sentsanurgentunmetclinicalneed.[3,7i,45] Waksman, isolated streptomycin from the soil microbe Soon after the discovery of chlorotetracycline and oxy- Streptomyces griseus. Just as they had done with penicillin tetracycline, Lloyd Conover at Pfizer discovered that the afewyearspreviously,pharmaceuticalchemistsimmediately carbon-chlorinebondofchlorotetracyclinecouldbecleaved begantoprobethestructureandpropertiesofstreptomycin. bycatalytichydrogenolysis,producingthefirstsemisynthetic In1946,RobertPeck,CharlesHoffhine,andKarlFolkersat tetracyclineantibiotic—tetracyclineitself(Scheme3).[46]The Merck[40]andQuentinBartz,JohnControulis,HarryCrooks, name “tetracycline” is derived from the four linearly fused, and Mildred Rebstock at Park, Davis & Co.[41] separately six-memberedringsthatarecommontoallmoleculesinthis discovered that catalytic hydrogenation of streptomycin family. Subsequently, tetracycline was found to be a natural produced a new compound, dihydrostreptomycin, which product,[47]andbytheendofthe1950stetracyclinewasthe exhibitedsimilarantibacterialpropertiesbutgreaterchemical mostprescribedbroad-spectrumantibioticintheU.S. stability (Scheme3). In 1950, U.S. pharmaceutical firms Although the chemical innovations that enabled the producedalmost100tonsofstreptomycinanddihydrostrep- discovery of dihydrostreptomycin and tetracycline may tomycin combined, as both antibiotics rapidly found clinical appeartrivialtoday,theyhadaseismicimpactonthestrategic applications.[42]Clinicaluseofthesedrugsinhumanswaslater mindsetofantibioticsdiscoveryandpharmaceuticaldevelop- discontinued as a result of their ototoxicity, though they ment more broadly. These innovations demonstrated that continuetobeusedinveterinarymedicine. naturalproductscouldbeconsideredasstartingpointsforthe discovery process—extremely useful but not necessarily optimal molecular scaffolds—and henceforth scientists in industryandacademiapursuedantibioticresearchwithequal vigor on two fronts: screening of soil samples for new antibacterial natural products, and chemical modification of natural antibiotics to find semisynthetic derivatives with improved therapeutic properties and patentable chemical structures.[29] Semisynthetic innovations have enabled dra- matic improvements in antibiotic therapy across all major families of natural antibiotics—here we will discuss the key events and chemical insights that helped overcome the numerous (and constantly evolving) limitations of cephalo- sporin,tetracycline,andmacrolideantibacterials. 3.2.Semisynthesisofb-LactamAntibiotics Scheme3. Theoriginsofantibacterialsemisynthesis. Theproducingstrainofthefirstcephalosporinantibiotics was discovered in 1948 by Giuseppi Brotzu, Professor of Hygiene at the University of Cagliari. Brotzu observed the The first tetracycline antibiotic was discovered in 1948, propensityoflocalsewageforself-purificationandhypothe- when Benjamin Duggar of Lederle Laboratories isolated sized that microorganisms were responsible. He studied the chlorotetracycline (Aureomycin) from the culture broth of microorganisms present at the outlet of a sewage pipe and Streptomyces aureofaciens,[43] and within two years Pfizer discovered that cultures of the mold Cephalosporium acre- scientists had isolated a second natural tetracycline, oxy- monium contained one or more substances that were 8846 www.angewandte.org (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim Angew.Chem.Int.Ed.2014,53,8840–8869 Angewandte Antibacterial DrugDiscovery Chemie antagonistictobacteria.Brotzufailedtoarouseinterestinhis discovered a compound that was soon to become (and discoveryintheItalianpharmaceuticalindustry,andhisdata remains to this day) the key semisynthetic intermediate for and a sample of Cephalosporium acremonium eventually production of cephalosporin antibiotics. A few years later, madetheirwaytoEdwardAbrahamatOxford.[21] RobertMorinandBillJacksonatEliLillydevelopedanovel In1955,EdwardAbrahamandGuyNewton,twochemists chemical method to remove the side chain of cephalospor- whoworkedwithFlorey,succeededinpurifyingcephalospor- inC,providingsemisynthetic7-ACAinacommerciallyviable inC from the Cephalosporium culture.[48] Abraham, like yield(40%).[50] others,hadobservedthesubtlyormarkedlydifferentproper- The synthesis of 6-aminopenicillanic acid (6-APA, Shee- ties of structurally distinct natural b-lactams and was han,1958)andsemisynthesisof7-aminocephalosporanicacid extremely interested in making chemical modifications to (7-ACA, Abraham & Newton, 1959), and the ensuing cephalosporins. Regarding cephalosporinC, he later development of practical methods for their preparation by recounted: “There was a great incentive to modify the fermentation (6-APA, Beecham Research Laboratories, molecule chemically with a view to increasing its intrinsic 1959)andbysemisynthesis(7-ACA,EliLilly,1962),respec- activity without affecting its resistance to staphylococcal tively, opened up the richest treasure trove of antibiotics in penicillinase.”[38c] (Penicillinase is a type of b-lactamase with human history. More than fifty commercial antibiotics have specificity for penicillins; b-lactamases are a collection of beendiscoveredandmanufacturedbychemicalmodifications bacterialenzymesthathydrolyticallyopentheb-lactamring, of semisynthetic 6-APA and 7-ACA. Here we will limit our producing inactive molecules). By 1959 Abraham and discussiontocephalosporins,describingthefavorableproper- Newtonhadsynthesizedsmallquantitiesof7-aminocephalo- ties that have been engineered into successive generations sporanic acid (7-ACA) by hydrolysis of cephalosporinC through 50years of human chemical evolution. This evolu- underacidicconditions(Scheme4).[49]Althoughtheyieldof tionary process began with cephalosporins that exhibited thisreactionwastoolowforcommercialproduction,theyhad usefulactivityagainstGram-positivebacteriaaloneandledto the development of compounds that are active against both Gram-positiveandGram-negativeorganisms(Figure3).This transitionishighlynoteworthyandisdiscussedagainlaterin thisReview. First-generation parenteral cephalosporins such as ceph- alothin(EliLilly,approved1964)[51]exhibitedpotentactivity against Gram-positive organisms but only moderate activity against Gram-negative bacteria. Since the 1960s, chemists have been able to synthesize compounds that possess a broader spectrum of activity, better pharmacological properties, as well as lower susceptibility to resistance mechanisms by introducing innovative side chains at just two modifiable sites of 7-ACA—the amine function at C7, and C3’ (see Figure3). The expanded-spectrum, second- generation cephalosporins tended to be somewhat less effective against Gram-positive bacteria but significantly Scheme4. Semisynthesisof7-aminocephalosporanicacid(7-ACA) moreactiveagainstGram-negativebacteria,owingtobetter fromcephalosporinC. cell penetration and resistance to b-lactamases. Importantly, Figure3. Humanchemicalevolutionofsemisyntheticcephalosporinantibiotics(definingstructuralfeaturesofeachgenerationarehighlightedin blue). Angew.Chem.Int.Ed.2014,53,8840–8869 (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim www.angewandte.org 8847 Angew.andte A.G. Myers etal. Reviews the a-methoxyimino group first introduced in cefuroxime 3.3.SemisynthesisofTetracyclineAntibiotics (Glaxo, approved 1983)[52] reduced susceptibility to b-lacta- mases by sterically blocking cleavage of the b-lactam ring. Beginning with Conover(cid:2)s landmark semisynthesis of Gram-negative activity was further improved in third-gen- tetracycline from chlorotetracycline, the development of erationcephalosporinssuchasceftazidime(Glaxo,approved semisynthetic tetracyclines has been marked by a series of 1985).[53] Ceftazidime incorporated an aminothiazole oxime specific, impactful discoveries. Charles Stephens and collab- with a charged carboxylate side chain, a combination that oratorsatPfizerachievedamajorenablingadvanceapprox- enhanced penetration through the porins embedded in the imately10yearsaftertheclasshadbeenidentifiedwhenthey outermembraneofGram-negativebacteriaandhelpedretain demonstrated in 1958 that the C6-hydroxy group of the high affinity for the bacterial target (penicillin binding natural products oxytetracycline, tetracycline and 6-deme- proteins). The emergence of b-lactamases that cleaved thyltetracyclinecouldberemovedreductively(Scheme5).[57] third-generation cephalosporins led to the development of The 6-deoxytetracyclines that arose as a consequence were fourth-generationmoleculessuchascefipime(Bristol-Myers found to be more stable than the parent compounds, yet Squibb, 1994),[54] which were more active than many third- retained broad-spectrum antibacterial activity. Their generation cephalosporins against both Gram-positive and enhancedchemicalstabilityenabledfurtherstructuralmodi- Gram-negative pathogens, including Pseudomonas aerugi- fications that had not been possible with acid- and base- nosa.[55] The fifth-generation cephalosporin ceftobiprole sensitive natural tetracyclines, leading to the discovery of received approval for use in Europe in 2013 for treatment minocyclinein1967byMichaelMartell,Jr.andJamesBoothe ofhospital-acquiredpneumonia.[56] at Lederle laboratories.[57b,58] Minocycline was synthesized The human chemical evolutionof cephalosporins vividly from6-deoxy-6-demethyltetracycline(sancycline)byanelec- illustrates the ability of medicinal chemists to continuously trophilicaromaticsubstitutionreactionatC7,anditexhibited tailorthepropertiesofantibacterialstomeetspecificclinical a broader spectrum of activity than prior tetracyclines needs.Widespreadclinicaluseofcephalosporinsandotherb- (including activity against some tetracycline-resistant staph- lactam antibiotics has selected for bacteria with fierce ylococci).Likeothermembersofthefamily,theclinicalutility collections of resistance determinants, but cephalosporins of minocycline declined in the ensuing decades due to remaincriticalcomponentsofourantibioticarmamentarium. increasinglywidespreadresistance. Of the seven drugs currently in advanced clinical develop- Aiming to overcome tetracycline resistance in the late ment(phaseIIorIII)forthetreatmentofinfectionscaused 1990s, a team of Wyeth scientists led by Frank Tally by Gram-negative bacilli, three are combinations of a ceph- synthesized7,9-disubstitutedtetracyclinederivatives,leading alosporin and a b-lactamase inhibitor.[3] Only one of these to the discovery of the life-saving antibiotic tigecycline (US new combination therapies (ceftolozane/tazobactam, Cubist approval 2005, Scheme5).[59] Tigecycline is the defining Pharmaceuticals) incorporates a novel cephalosporin anti- member of a new class of tetracyclines known as glycylcy- biotic, suggesting that the development of new b-lactams is clines, which greatly extend the spectrum of tetracyclines, becomingincreasinglydifficult. especiallytowardtetracycline-resistantmicroorganisms.Tige- Scheme5. Chemicalinnovationsintetracyclinesemisynthesis(importantnewstructuralfeaturesofeachgenerationarehighlightedinblue). 8848 www.angewandte.org (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim Angew.Chem.Int.Ed.2014,53,8840–8869 Angewandte Antibacterial DrugDiscovery Chemie cycline has become a last line of defense against multidrug- metabolicproductsfromStreptomycesthatcontainamacro- resistantbacteria;forexample,itisoneofonlytwoapproved lactone ring.[62] Erythromycin was approved for use against antibioticsthatareactiveagainstsomecarbapenem-resistant a variety of Gram-positive bacterial infections, but upon bacteria carrying New Delhi metallo-b-lactamase enzymes widespread clinical implementation several limitations were (NDMs, see Section4.3 for discussion of carbapenem anti- quickly identified. Erythromycin displayed poor oral bio- biotics).[5] The other is colistin, which can cause damage to availabilityandashortinvivohalf-life,andmostimportantly kidneys and nerves. Some of the benefits of tigecycline are itwasfoundtobeunstableunderacidicconditions,givingrise attributabletothefactthatitbindsmorestronglythanolder to side effects such as stomach pain. Administration of the tetracyclines to the small subunit of the bacterial ribosome antibiotic as an enteric-coated tablet helped sidestep insta- (the biological target of all tetracyclines).[60] Its drawbacks bilitytogastricacid;however,innovativechemicalsolutions include dose-limiting tolerability (nausea, vomiting) and were much desired. Studies of chemical instability under alackoforalbioavailability.Thehumanchemicalevolution acidicconditionsrevealedthaterythromycindecomposesby of semisynthetic tetracyclines has provided antibacterial intramolecular cyclization reactions beginning with addition therapies that have overcome many limitations of their of the C6 hydroxy group to the C9 ketone, leading to predecessors, but the slowing pace of discovery in this area formation of both anhydrohemiketal and spiroketal deriva- isevident(Figure4). tives (Scheme6).[63] Knowledge of the chemical basis for instability catalyzed the discovery of semisynthetic macro- lidesthatlackedthissignificantlimitation. 3.4.SemisynthesisofMacrolideAntibiotics One solution was provided by Sadafumi Omura and collaborators at Taisho Pharmaceutical Co. in Japan who Macrolide antibiotics have undergone serial human developed a 6-stepsequence from erythromycin resulting in chemical evolutionary advances that in many ways parallel selectivecappingoftheC6hydroxysubstituentwithamethyl those that occurred within tetracycline antibiotics: each group, affording the antibiotic clarithromycin (Scheme6). semisynthetic advance has built upon prior innovations, and ProtectionoftheC9ketoneoferythromycinasanoximewas each new (successful) semisynthetic antibiotic has become critical to this work, providing an intermediate whose a starting material for further chemical modification conformation enabled selective methylation at C6-OH.[64] (Figure4).[61] This strategy is sensible, since it enables Clarithromycin displayed a slightly expanded spectrum of favorable characteristics to be carried forward, but it also activityrelativetoerythromycin,anditwasfoundtobeboth inevitably leads to a gradual increase in the number of acid-stableandorallyactive. chemical operations required to synthesize new derivatives Another innovative semisynthetic solution to the chem- fromtheoriginalnaturalproduct. ical instability of erythromycin was developed in 1980 by Erythromycin, the first macrolide antibiotic, was discov- GorjanaLazarevzkiandco-workersatPlivainCroatia.Inthis ered in 1949 when scientists at Eli Lilly isolated the natural case, the C9 ketone was completely removed from the product from the culture broth of the soil-dwelling fungus erythromycin scaffold by a sequence comprising oxime Saccharopolyspora erythrea. The term “macrolide” was formation, Beckmann rearrangement (ring expansion), and originallyintroducedbyR.B.Woodwardin1957todescribe thenhydrogenolysisoftheresultingiminoetherintermediate Figure4. Humanchemicalevolutionoftetracyclineandmacrolideantibioticsbysemisynthesis(importantnewstructuralfeaturesofeach generationarehighlightedinblue). Angew.Chem.Int.Ed.2014,53,8840–8869 (cid:2)2014Wiley-VCHVerlagGmbH&Co.KGaA,Weinheim www.angewandte.org 8849
Description: