Systematic Revision Within the Phalanger orientalis Complex (Diprotodontia, Phalangeridae): A Third Species of Lowland Gray Cuscus from New Guinea and Australia PDF
Preview Systematic Revision Within the Phalanger orientalis Complex (Diprotodontia, Phalangeridae): A Third Species of Lowland Gray Cuscus from New Guinea and Australia
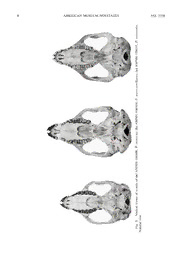
PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, NY 10024 Number 3356, 20 pp., 8 figures, 3 tables December 31, 2001 Systematic Revision Within the Phalanger orientalis Complex (Diprotodontia, Phalangeridae): A Third Species of Lowland Gray Cuscus from New Guinea and Australia CHRISTOPHER A. NORRIS1 AND GUY G. MUSSER2 ABSTRACT A study of specimens from the mammal collections of the American Museum of Natural History that were originally assigned to Phalanger orientalis sensu lato revealedthepresence of three taxa: P. orientalis from northern New Guinea, the Bismarck Archipelago, and the SolomonIslands;P.intercastellanusfromsoutheastNewGuinea,theD’EntrecasteauxIslands, the Trobriand Islands, and the Louisade Archipelago; and agroup fromsouthern NewGuinea and the Cape York Peninsula of Australia that could not be assigned to either of the two foregoing species. Possession of a distinctive suite of morphological characters warrants rec- ognition of this group at the species level. The characteristics and habitat of the species are described, and the zoogeographic implications of its distribution are discussed. INTRODUCTION phylogentic revision of the cuscuses, by Tate (1945), placed all species in a single genus, The taxonomy of the lowland gray cus- Phalanger,whichwassplitintothreespecies cuses, small- to medium-sized arboreal mar- groups. The largest of these, the ‘‘orientalis supialsofthefamilyPhalangeridae,hasbeen group’’,contained fourspecies:P.orientalis, in a state of flux for many years, a situation P. gymnotis, P. vestitus, and P. celebensis. that was summarized in somedetailbyMen- The first of these species, P. orientalis, was ziesandPernetta(1986).Thefirstlarge-scale divided into five subspecies, subsumedwith- 1DivisionofVertebrateZoology (Mammalogy),AmericanMuseumofNaturalHistory.e-mail:[email protected] 2DivisionofVertebrateZoology(Mammalogy),AmericanMuseumofNaturalHistory.e-mail:[email protected] Copyright(cid:113)AmericanMuseumofNaturalHistory2001 ISSN0003-0082 2 AMERICAN MUSEUM NOVITATES NO. 3356 in which were a number of taxa previously of the central cordillera between the Kikori considered to be of specific rank. These in- and Mimika rivers and encompassing low- cluded P. ornatus (Gray, 1860), P. lullulae land rainforests, seasonally flooded forests, (Thomas, 1896), P. vulpecula (Fo¨rster, openwoodlands,andtropicalgrasslands.The 1913), P. brevinasus, P. matsika, and P. mi- absence of specimens is perhaps unsurpris- crodon (Tate and Archbold, 1935). Subse- ing, as the area has rarely been visited by quent studies (George, 1979, 1982; Ziegler, Western collectors. Nonetheless, the paucity 1982; Menzies and Pernetta, 1986) resur- of material is frustrating, as the few speci- rected some of these groups as full species, mens of P. orientalis sensu lato collected but Tate’s basic concept of a single,morpho- here are distinctive enough to have been de- logically diverse species, encompassing cus- scribedasaseparatesubspecies(P.orientalis cuses from the lowlands of New Guinea, to- mimicus, Thomas, 1922), a separate species gether with those of the Solomon Islands, (P. microdon, Tate and Archbold, 1935), or Bismarck Archipelago, Louisade Archipela- even assigned to a different genus (Strigo- go, D’Entrecasteaux Islands, Trobriand Is- cuscus mimicus, Flannery et al., 1987). The lands, South Moluccas, Timor, and the Cape results of a discriminant function analysis of York Peninsula of Australia, has persisted in metric characters by Menzies and Pernetta the literature. (1986)revealboththecleardistinctionofthe In recentyearsithasbecomeclearthatthe southern New Guinea population of P. or- apparently homogenous P. orientalis ofNew ientalis from the other New Guinea popula- Guinea, Cape York, and nearby islands may tions and its affinities with the Cape York contain within it several populations suffi- population. On this basis, Menzies and Per- ciently distinctto warrantrecognitionasspe- netta recognized the two populations as a cies in their own right. Menzies andPernetta separate subspecies, P. orientalis mimicus. (1986) identified seven populations on the TheAmericanMuseumofNaturalHistory basis of palatal dimensions and non-metric possesses a large series of phalangerid mar- morphological characters, but declined to supialsresultingfromtheeightArchboldEx- recognize these as anything more than sub- peditions of 1933 to 1964 to New Guinea species of P. orientalis. An electrophoretic and the Cape York Peninsula of Australia study by Colgan et al.(1993)showedtheex- (Archbold and Rand, 1935; Rand and Brass, istenceof twopopulationswithinNewGuin- 1940; Archbold et al., 1942; Brass, 1953, ea that were sufficiently divergent to suggest 1956, 1959, 1964; Van Deusen, 1978). Re- they represented two species: P. orientalisin curation of these collections by Musser and northern New Guinea and the Bismarck and Helmut Sommer in the 1990s provided an Solomon Islands, and P. intercastellanus in opportunity to reassess the taxonomy of the southern New Guinea and the southeastPap- lowland gray cuscuses. It became apparent uanIslands.Theresultsoftheelectrophoretic that while the majority of P. orientalissensu study were supported by an analysis of mor- lato specimens could be assigned to eitherP. phological characters, which can be used to orientalissensustrictoorP.intercastellanus, distinguish between the two species (Colgan there remained a set of specimens, collected et al., 1993). Colgan et al.’s combination of molecular in the southern lowlands of New Guinea and andmorphologicalevidencefortheexistence the Cape York Peninsula of Australia,whose of two species within Phalanger orientalis distinctive morphological characteristics fit- sensu lato is compelling. However, the spec- ted into neither of the species described by imens used as the basis of the study are Colgan et al. (1993). This report describes drawn from a comparatively limited sample, these specimens, and forms part of series of taken primarily from offshore islands and a reports, either published (Musser and Som- restricted number of sites in mainland New mer, 1992) or in preparation, that reflect the Guinea. There are no specimens from the Department of Mammalogy’s efforts to gain southern lowlands of New Guinea(including a better understanding of the species diver- the area known as the Trans-Fly),aregionof sity and zoogeography of mammals in New around125,000squaremilesextendingsouth Guinea. 2001 NORRIS AND MUSSER: PHALANGER ORIENTALIS COMPLEX 3 Fig. 1. Collecting localities for specimens of Phalanger intercastellanus, P. mimicus, and P. orien- talis in the AMNH collections, and for non-AMNH specimens used in the description of P. mimicus. Solomon Islands and Australian localities are not shown on the map. For details of all localities see appendix 1. Locality 33 ((cid:53) ‘‘New Britain’’) is not shown because it is nonspecific. MATERIALS AND METHODS diagnosticcharactersdefinedbyColganetal. (1993) resulted in the species distribution Specimens of Phalanger orientalis sensu seen on the map, with a clear zone of sepa- lato from the American Museum of Natural ration at the watershed of the Markham/ History, Department of Mammalogy Ramu Valley. Specimens of both species (AMNH);theNaturalHistoryMuseum,Lon- don, Mammal Collections (BMNH); and the were collected at Umi River Camp (fig. 1, Bernice P. Bishop Museum (BBM) were ex- localities 1 and 34) during the Sixth Arch- amined. A list of specimens is attached as bold Expedition to New Guinea, although appendix 1. Measurements were taken using this should not be taken as evidence of sym- vernier calipers. Becausethecraniumofcus- patry. The expedition mammalogist Hobart cusescontinuestogrowthroughoutadultlife Van Deusen frequently obtained specimens (MenziesandPernetta,1986)onlyspecimens from local hunters, which could have been in which M4 was fully erupted were mea- brought to the camp from either side of the sured. Dental terminology follows Luckett Markham/Ramu watershed. Dimensions for (1993). the skulls of mainland New Guinea popula- tions of P. intercastellanus and P. orientalis RESULTS are given in table 1. The AMNH collections contain 1162 A third group of specimens, from the specimens assigned to Phalanger orientalis Western Province of Papua New Guinea, sensu lato. The main collecting localities for could be distinguished from both P. orien- these are listed in appendix 1 and are shown talis and P. intercastellanus by their smaller in figure 1. Assignment of specimens from overall size; their particularly small molars; mainland New Guinea to P. orientalis sensu the presence of a well-developed postpara- stricto and to P. intercastellanus, using the cristaonM1,withapronouncedbuccalkink; 4 AMERICAN MUSEUM NOVITATES NO. 3356 TABLE1 Descriptive Statistics for Cranial and Dental Measurements (mm) for New Guinea Population Samples of Phalanger orientalis and Phalanger intercastellanus (Mean and observed range [in parentheses] are listed.) the reddish-brown pelageofthefemales;and ther P. orientalis (BismarckArchipelagoand most strikingly by the distinctive alignment SolomonIslands)orP.intercastellanus(East of the zygomatic arches, which are parallel Papuan Islands). However, the Cape York to the midline of the skull or diverge ros- population showed clear affinities with the trally, rather than converging rostrally. Skull WesternProvincespecimens,includingsmall dimensions for these specimens are listed in size of molars and the alignment of the zy- table 2. gomaticarches,althoughfemalesofthispop- The study was then expanded to include ulation lacked the reddish pelage of the New specimensfromtheEastPapuanIslands,Bis- Guinea females. Cranial dimensions of this marck Archipelago, Solomon Islands, and population are listed in table 2. the Cape York Peninsula of Australia. Al- Examination of the holotype of P. orien- though significantly smaller than the main- talis mimicus Thomas, 1922, in the collec- land populations of the two species defined tions of the Natural History Museum, Lon- by Colgan et al. (1993), it was possible to don, revealed that it possessed the same dis- assign the majority of these specimens to ei- tinctive cranial charactersseenintheAustra- 2001 NORRIS AND MUSSER: PHALANGER ORIENTALIS COMPLEX 5 TABLE2 Descriptive Statistics for Cranial and Dental Measurements (mm) for New Guinea and Australian Population Samples of Phalanger mimicusa (Mean and observed range [in parentheses] are listed.) lian and Western Province specimens at the Phalanger mimicus (Thomas, 1922) AMNH. They were also seen in three spec- Phalanger intercastellanus Thomas, 1895. imens of ‘‘P. orientalis’’ from the Bishop Phalanger orientalis mimicus Thomas, 1922. Museum, which were collected on the lower Phalanger microdon Tate and Archbold, 1935. slopes of Mount Bosavi, in the Southern Phalanger orientalis peninsulae Tate, 1945. Highlands Province of Papua New Guinea. HOLOTYPE AND TYPE LOCALITY: BMNH 11.11.11.93, puppet skin with skull and den- DISCUSSION taries of an adult male. Collected by G. C. Shortridge, Parimau, Mimika River, Dutch On the basis of the consistent morpholog- New Guinea ((cid:53) Irian Jaya), 4(cid:56)31(cid:57)S, ical differences between the southern New 136(cid:56)36(cid:57)E, 250 ft, 4 October 1910. Guinea and Cape York populations of Pha- DIAGNOSIS: Skull and teeth small, molars langer orientalis sensu lato, we recognize a especially so. Lachrymal contribution to the third species of lowland gray cuscus in the face is short. Zygomatic arches run parallel P. orientalis complex (fig. 2). or diverge from midline of skull rostrally. 6 AMERICAN MUSEUM NOVITATES NO. 3356 Fig. 2. Captive specimen of a female Phalanger mimicus. Third Archbold New GuineaExpedition, 1936–1937. Gaima, Western Province, Papua New Guinea. Well-developed postparacrista on M1, with a absence of lingual cingulum on protocone of pronounced buccal kink. Distinguished from M2. P. orientalis and P. intercastellanus by its DESCRIPTION: Critical skull dimensions are smaller size, lack of convergence of zygo- listed in table 2. The most notable feature of maticarchesrostrally,andbywell-developed the skull is the shape of the zygomatic arch- postparacrista. Distinguished from P. orien- es.InallotherspeciesofPhalangerthearch- talis by absence of white tail tip in females. es reach their widest point at the caudal end, Distinguished from P. intercastellanus by near the suture between the jugal and the 2001 NORRIS AND MUSSER: PHALANGER ORIENTALIS COMPLEX 7 squamosal, and converge toward the midline ing that, unlike the condition seen in Austra- of the skull rostrally. In P. mimicus thearch- lian P. mimicus, the dorsal stripe continues esrunparalleltothemidline,orevendiverge almosttothebaseofthetail,albeitbecoming rostrally, giving the skull a different shape more diffuse caudally. The question of (fig. 3). In general the skull shows less de- whether the differences in pelage between velopment of the supraorbital, parietal, and the Trans-Fly and Cape York populations sagittal crests than is seen in other speciesof warrant taxonomic recognition cannot be an- the genus; indeed, it is not unusual for the swered without a larger sample from south- parietal crests to remain unfused, even in west New Guinea. very old individuals. The facial extent of the HABITAT: Rand and Brass (1940) provide lachrymal is comparatively small, as in P. descriptions of the southern New Guinea re- intercastellanus.Thefrontalsaredeeplypen- gion that containsthecollectinglocalitiesfor etrated by the nasal bones; the nasals do not P.mimicus.Intheareaalongthecoast,south taper abruptly at their caudal extremity, but of the estuary of the Fly River (which in- arerounded(fig.4).Theteethareremarkably cludes the Oriomo River: fig. 1, locality 62), small, even given the overall small size of the predominant vegetation type is scrub sa- the animals. In this respect P. mimicus is vanna. Rainforest occurs chiefly as fringing similar to the rare montane Telefomin cus- strips of no more than 100–200 m in depth cus, P. matanim (Flannery, 1987). The well- along creeks and streams, and in isolated developedpostparacristaonM1,withitspro- patches on ridge tops (fig. 6). The rainforest nounced buccal kink (fig. 5), is seen in sev- is described by Rand andBrassas‘‘poorand eral species of Phalanger, but not in either light’’, with some areas that ‘‘could only be P. orientalis or P. intercastellanus, the two describedasdensebrush’’.Savannatrees,in- species within which P. mimicus was for- cluding Acacia and Tristania, intrude into merly reduced to synonymy. the rainforest. The savannas are dominated The tail wedge of P. mimicus is short, as by Melaleuca; on ridges this genus mixes in P. intercastellanus. Adult males of the with Tristania, Eucaplyptus, and Acacia to Australian population have upper parts that form savanna forests with a thin, high can- are gray, with a well-defined dark dorsal opy and a ground cover of grasses. During stripe finishing well short of the haunches, the rainy season the flat savannas become and silver-tipped guard hairs. Three speci- boggy, and can flood up to a foot (Brass and mens, including the holotypes of P. orien- Rand,1940).Incontrast,theareasaroundthe talis mimicus (BMNH 11.11.11.93) and P. Palmer River Camp (fig. 1, locality 63) and orientalis peninsulae (AMNH 108905),have the Black River Junction (fig. 1, locality 64) an off-white pelage with dark guard hairs. in the northern part of Western Province are This coloration is often seen in old males of typified by steep ridges rising up to 100 m both P. orientalis and P. intercastellanus. above sea level (Brass and Rand, 1940). The With the exception of aged specimens, how- vegetation type is classic lowland rainforest ever, P. mimicus appears to show little or no richinepiphytesinboththelowerlayersand sex-based color dimorphism, at least in its the canopy (fig. 7). Australian populations. The situation is less The Australian localities for P. mimicus clear for the New Guinea population, be- aredescribedbyBrass(1953),andbearstrik- cause only one adult male, the aforemen- ing similarities in vegetation to those seen in tioned BMNH 11.11.11.93, is known. Fe- southern New Guinea. At Shepheard’s Bat- males of the New Guinea population have a tery site, on the Upper Peach River (fig. 1, striking reddish-brown pelage, which was locality60),Brassreportedthatspecimensof one of the characteristics used by Tate to de- P. mimicus were ‘‘found in numbers’’ in the scribeP.microdon.Juvenilemalesalsoshow rainforest fringing Bonanza Creek. Rainfo- this coloration, but given the generaltenden- restwasconfinedtothemarginsofthecreek, cy toward a reddish pelage in juveniles of while the remainder of the flats and sur- both P. orientalis and P. intercastellanus, rounding ridges were covered inopensavan- this may be of limited significance in distin- na forests of box, bloodwood, and ironbark guishingbetweenthespecies.Itisworthnot- trees. In the Iron Range (fig. 1, locality 59) 8 AMERICAN MUSEUM NOVITATES NO. 3356 s. ali nt e ri o P. 5, 3 4 9 0 1 H N M A ) c ( s; u n a ell st a c r e nt i P. 9, 6 5 8 0 1 H N M A ) b ( s; u c mi mi P. 0, 0 4 4 0 1 H N M A ) a ( f o a ni a r c f o s w e vi al r nt e Ve. z 3.si g.ral Fiatu N 2001 NORRIS AND MUSSER: PHALANGER ORIENTALIS COMPLEX 9 s. ali nt e ri o P. 5, 3 4 9 0 1 H N M A ) c ( s; u n a ell st a c r e nt i P. 9, 6 5 8 0 1 H N M A ) b ( s; u c mi mi P. 0, 0 4 4 0 1 H N M A ) a ( f o a ni a r c f o s w e vi al s r o De. z 4.si g.ral Fiatu N 10 AMERICAN MUSEUM NOVITATES NO. 3356 Fig. 5. Right molar tooth rows of (a) AMNH 104400, P. mimicus; (b) AMNH 108569, P. inter- castellanus;(c)AMNH109435,P.orientalis.Abbreviations:lc(cid:53)lingualcingulumofM2;bk(cid:53)buccal kink in postparacrista of M1. Approximately 3.5 (cid:51) natural size. lowland rainforests predominated (fig. 8), the other New Guinea populations and, sec- though therewere stillpatchesofopenforest ond, its affinities with the Cape York popu- in which rainforest was limitedtocreekmar- lation. On this basis, Menzies and Pernetta ginsandgullies.AswiththeFlyRiverbasin, recognized the two populations as a separate there was seasonal inundation of the rainfo- subspecies, P. orientalis mimicus. Our study rests on the floodplains, in some areas to supportstheresultsoftheiranalysis,theonly depths of 6–10 ft (Brass, 1953). difference being their retention of mimicus COMMENTS:Anumberofauthorshaverec- within P. orientalis. Except for its consis- ognized the distinctive nature of the lowland tently smaller size, Menzies and Pernetta gray cuscuses from southern New Guinea could find no distinctive characters to sepa- (Thomas, 1922; Tate and Archbold, 1935; rate the taxa. In fact, as we havedemonstrat- Menzies and Pernetta, 1986; Flannery et al., ed, the qualitative differences in cranialmor- 1987; George, 1987). Particularly notewor- phology between mimicus and theotherpop- thy among these earlier studies is that of ulationsofP.orientalissensulatoareatleast Menzies and Pernetta (1986), who used a as great as those that define a number of discriminantfunctionanalysisofmetricchar- well-established species of phalangerids,and acters(largelydrawnfromthepalatalregion) arecertainlysufficienttojustifyseparationat to distinguish different populations of P. or- the species level. ientalis sensu lato. Their analysis revealed The systematicplacementofP.mimicusis first, the clear distinction of the southern problematic. The characteristics that were New Guinea population ofP.orientalisfrom originally proposed by Flanneryetal.(1987)
