Signal Transduction in Plants PDF
Preview Signal Transduction in Plants
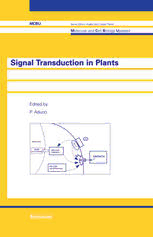
MCBU Molecular and Cell Biology Updates Series Editors: Prof. Dr. Angelo Azzi Institut fur Biochemie und Molekularbiologie BOhlstr.28 CH-3012 Bern Switzerland Prof. Dr. Lester Packer Dept. of Molecular and Cell Biology 251 Life Science Addition Membrane Bioenergetics Group Berkeley, CA 94720 USA Signal Transduction in Plants ~dited by P. Aducci Birkhauser Verlag Basel· Boston· Berlin Volume editors' address: Prof. P. Aducci University of Rome "Tor Vergata" Department of Biology Via della Ricerca Scientifica 1-00133 Rome Italy A CI P catalogue record for this book is available from the Library of Congress, Washington D.C., USA Deutsche Bibliothek Cataloging-in-Publication Data Signal transduction in plants / ed. by P. Aducci. -Basel; Boston; Berlin: Birkhiiuser, 1997 (Molecular and cell biology updates) ISBN-13: 978-3-0348-9938-3 NE: Aducci, Patrizia IHrsg.1 The use of registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher cannot assume any legal responsibility for given data, especially as far as directions for the use and the handling of chemicals and drugs are concerned. This information can be obtained from the manufacturers. This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically those of translation, reprinting, re-use of illustrations, broadcasting, reproduction by photocopying machine or similar means, and storage in data banks. Under § 54 of the German Copyright Law, where copies are made for other than private use, a fee is payable to "Verwertungsgesellschaft Wort", Munich. © 1997 Birkhiiuser Verlag, PO Box 133, CH-4010 Basel, Switzerland SofIcover reprint of the hardcover 1s t edition 1997 Printed on acid-free paper produced from chlorine-free pulp. ISBN-13: 978-3-0348-9938-3 e-ISBN-13: 978-3-0348-9183-7 001: 10,1007/978-3-0348-9183-7 987654321 Table of Contents Preface ................................................... VII Chemical signals: Hormones, Phytotoxins Roles of ion channels in initiation of signal transduction in higher plants I.M. Ward and 1.1. Schroeder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 ABA signaling in plant development and growth T.L. Thomas, H.-I. Chung and A. N. Nunberg .......................... 23 Auxin perception and signal transduction M.A. Venis and R.M. Napier ..................................... 45 Transduction of ethylene responses M.A. Hall and A.R. Smith . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65 Phytotoxins as molecular signals P. Aducci, A. Ballio and M. Marra . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83 Physical signals: Blue and red light Blue light-activated signal transduction in higher plants W.R. Briggs and E. Liscum ...................................... 107 The transduction of light signals by phytochrome C. Bowler ................................................. 137 Biotic signals: Host-pathogen interactions Perception of fungal elicitors and signal transduction F. Cervone, R. Castoria, F. Leckie and G. De Lorenzo 153 Subject index ...... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 179 Preface Increasing interest has been emerging in the last decade in the field of signal recognition and transduction. This is particularly true for animal systems where an impressive amount of literature is appearing and where many important pathways have been clarified at a molecular level. In the elucidation of the functions of single components of a given pathway, gene cloning has played a major role and opened the field to the genetic engineering of these complex systems. At variance with this situation, plant systems are less well elucidated, even if in recent years exciting research developments have been initiated especially with the view toward the most promising role of plants in biotechnology. Recent studies have elucidated some of the events involved in the perception of the plant hormone signals and some steps concerning its transduction. Only for three of the five hormones in plants, namely auxin, ethylene and cytokinins, have specific receptors been isolated. The use of classical molecular approaches, together with the more recently isolated mutants, have produced crucial information on receptors and shed light on possible transduction pathways. As in the case of red light, more than one pathway can be triggered by one specific signal. Many systems involved in animal signaling are now shown to be present also in plants, and in view of the fast progress in this area, it will be possible in the near future to fully describe the content of the "black boxes" in the reaction chain specifically triggered by a signal. These have been some of the reasons that prompted us to prepare the present book. It reflects the current "state of the art" in the field of signal transduction in plants. The contributing authors are experts in different areas of plant physiology and plant molecular biology and have used different approaches to study the recognition and transduction of different signals specific for plant organisms. These can be chemical or physical in nature and some examples will be presen ted on the molecular mechanisms by which hormones, toxins or light can regulate plant growth, differentiation and morphogenesis. Moreover, plant-pathogen interactions are now beginning to be clarified and some progress on their molecular basis is being described. This book, for its multidisciplinary approach, for the different technologies used and for the emerging interesting peculiarities of the plant world should be of interest both to experts in the field and to a wider audience. I would like to thank my coworkers Vincenzo Fogliano, Maria Rosaria Fullone, Mauro Marra and Silvia Bacocco for their help in organizing the material for the book and the staff of Birkhauser for their collaboration. Patrizia Aducci Signal Transduction in Plants P. Aducci (ed.) © 1997 Birkhiiuser Verlag BaseVSwitzerland Roles of ion channels in initiation of signal transduction in higher plants I.M. Ward and 1.1. Schroeder Department of Biology and Center for Molecular Genetics, University of California San Diego, La Joila, CA 92093-0116, USA Introduction Recently, progress has been made in identifying initial signal reception mechanisms and early events in signaling cascades in higher plants. Ion channels, along with intracellular signaling pro teins and second messengers, are critical components mediating early events in higher plant signal transduction. Ion channel-mediated signal transduction in higher plants has notable differences from signaling mechanisms in animal systems. Of the many types of ion channels found in higher plants, there are indications that anion channels, along with Ca2+ channels, play critical and rate-limiting roles in the mediation of early events of signal transduction. We have now begun to obtain the first insights into the modes of regulation, the membrane localization, and in the case of K+ channels, the molecular structure of higher plant ion channels. In this chapter we focus mainly on novel findings concerning the function and regulation of anion and Ca2+ channels and outline testable models of their involvement in signal transduction. Our objective is to summarize these findings and to point out the many open questions involving early events in plant signal transduction. To illustrate the functions of higher plant ion channels in the initiation of signaling cascades, in the first section we discuss the relatively better understood molecular mechanisms of abscisic acid (ABA)-induced stomatal closing with a special focus on new and emerging concepts. In the second section, we address Ca2+-dependent and Ca2+-inde pendent signaling processes in plants and analyze certain putative parallels between initial guard cell signaling and both the initiation of defense responses and phytochrome-induced signaling. Abscisic acid-initiated signal transduction in guard cells Since the initial patch-clamp studies of guard cells showed a role for K+ channels in mediating stomatal movements (Schroeder et aI., 1984), guard cells have become a model system for under- 2 J. M. Ward and J. 1. Schroeder standing early signal transduction events in plants. A variety of signals, including hormones, light, humidity, and water status, influence stomatal aperture, allowing plants to balance CO2 uptake and water loss under diverse environmental conditions. The hormone ABA is synthesized in response to drought and induces a cascade of signaling events in guard cells reSUlting in stomatal closing (Mansfield et a!., 1990). Recent research has led to the identification of several early events in ABA signaling, providing a potent system to analyze the intermediate steps in this transduction cascade. The events that directly follow ABA receptor activation have proven difficult to identify, primar ily because of a general lack of information concerning the cellular location and structure of the ABA receptor. Experiments involving microinjection of ABA have indicated a requirement for extracellular ABA receptors (Anderson et aI., 1994; Gilroy and Jones, 1994). However, the pH dependence of ABA inhibition of stomatal opening (Anderson et a!., 1994, and references therein), as well as the effects of the release of caged ABA within guard cells (Allan et a!., 1994) and the effects of microinjected ABA on stomatal closing in the presence of 1 ~M extracellular ABA (Schwartz et a!., 1994) argues for a role of cytosolic ABA in guard cell signaling. In addi tion, ABA-induced 86Rb+ efflux from guard cells appears to depend on the concentrations of A B c channel D K + channel Figure 1. Simplified model for the coordinated regulation of plasma membrane ion channels required for ABA-in duced stomatal closing. (A) Activation of abscisic acid receptors leads to Ca2+-dependent and Ca2+-independent signaling events that regulate ion channel activity. (B) The rapid activation of nonselective ion channels causes membrane depolarization and allows Ca2+ influx from the extracellular space. The increase in cytosolic Ca2+ and the production of other signaling intermediates trigger further Ca2+ release from intracellular stores. (C) Membrane depolarization, elevated cytosolic Ca2+, and protein phosphorylation events activate anion channels, which mediate anion release and long-term membrane depolarization. (D) K+ efflux through voltage dependent outward K+ chan nels is driven by membrane depolarization and enhanced by ABA-induced cytosolic alkalinization. Roles of ion channels in initiation of signal transduction in higher plants 3 both intracellular and extracellular ABA (MacRobbie, 1995). A model that may account for these circumstantial observations has been proposed in which both extracellular and intracellular sites for ABA reception exist (Anderson et al., 1994; MacRobbie, 1995). A better understanding of the mechanism of ABA perception and the initial targets of activated receptors requires further cellu lar and molecular identification of the ABA receptors. One of the earliest known responses of guard cells to ABA is an increase in cytosolic Ca2+ (McAinsh et aI., 1990; Schroeder and Hagiwara, 1990). Increases in cytosolic Ca2+ have been demonstrated to be sufficient to induce stomatal closure (Gilroy et aI., 1990). The activation of non-selective, Ca2+ permeable ion channels in the plasma membrane that allow Ca2+ influx occurs within 2 seconds of ABA exposure (Schroeder and Hagiwara, 1990), indicating a close coupling between ABA receptors and these ion channels. The oscillatory activity of Ca2+ -perme able ion channels indicates a close but not direct coupling as depicted in Figure 1 (Schroeder and Hagiwara, 1990). Although it has been suggested that Ca2+ influx from the extracellular space is required for stomatal closing (DeSilva et aI., 1985; Schwartz et al., 1988), recent work has indi cated that Ca2+ is released in parallel from intracellular stores: injection of inositol 1,4,5-trisphos phate (InsP3), which is thought to trigger Ca2+ release from internal stores in guard cells, produ ces stomatal closing (Gilroy et aI., 1990). In fact, evidence indicates a possible increase in InsP3 levels in guard cells in response to ABA (MacRobbie, 1992; Cote and Crain, 1994). Cytosolic Ca2+ (Schroeder and Hagiwara, 1989) and InsP3 (Blatt et al., 1990) also reversibly inhibit inward K+ channels. Furthermore, the inhibition of inward-rectifying K+ channels in guard cells following ABA application is not dependent on extracellular Ca2+ but is abolished by intracellular application of Ca2+ buffers. These data suggest that ABA-induced Ca2+ release from intracellular stores can mediate K+ channel inhibition, while not excluding the occurrence of Ca2+ influx at the plasma membrane (Lemtiri-Chlieh and MacRobbie, 1994). In support of these findings sugges ting multiple cellular sources of Ca2+ influx into the cytosol, imaging studies have shown that ABA induces transient cytosolic Ca2+ increases in the vicinity of both the plasma membrane and the vacuole in guard cells (McAinsh et al., 1992). More recently, oscillations in cytoplasmic free Ca2+ in guard cells, which result in stomatal closure, have been directly demonstrated to depend on both Ca2+ influx and intracellular Ca2+ release (McAinsh et al., 1995). These data, as well as other findings on Ca2+- independent signaling (Allan et aI., 1994, discussed later in this chapter), indicate that guard cells provide a good system to analyze parallel signaling mechanisms. It is becoming evident that multiple Ca2+ channels exist in guard cells and the immediate questions of signal specificity and coupling mechanisms require further analysis. For example, it has not yet been directly demonstrated whether InsP3 induces Ca2+ influx from intracellular stores in guard cells or from the extracellular space. 4 J. M. Ward and J. 1. Schroeder Regulation of K+ channels and H+ pumps during guard cell signaling Increases in cytosolic Ca2+ inhibit cellular components that are important in controlling stomatal opening. Plasma membrane H+-ATPase activity that drives stomatal opening is inhibited almost completely when cytosolic Ca2+ is elevated to 111M (Kinoshita et al., 1995). The inhibition of H+ pumping would inhibit stomatal opening, but alone is unlikely sufficient to induce stomatal clo sure, as described below. Proton pump inhibition is not sufficient to generate the membrane depolarization positive of the K+ eqUilibrium potential required for stomatal closing. Inward-recti fying K+ channels in the guard cell plasma membrane are also inhibited by elevated cytosolic Ca2+ (Schroeder and Hagiwara, 1989; Blatt et aI., 1990; Fairley-Grenot and Assmann, 1991; Lemtiri-Chlieh and MacRobbie, 1994; Kelly et aI., 1995). Recent work demonstrated that strong ly elevated cytosolic Ca2+ levels in the range of I to 1.3 11M are required to significantly inhibit inward-rectifying K+ channels in guard cells (Kelly et aI., 1995). This regulation of K+ influx is considered to be of secondary importance for the stomatal closing mechanisms discussed here because K+ uptake channels provide a major pathway for K+ accumulation during stomatal opening (Schroeder et a!., 1987; Schroeder, 1988; Schroeder and Hagiwara, 1989; Thiel et al., 1992) and have not been proposed to contribute to stomatal closing. Recent findings on second messenger regulation of these K+ uptake channels are reviewed elsewhere (Assmann, 1993). Potassium channel currents in guard cells are much larger than required for physiological K+ fluxes during stomatal movements. Thus, inward K+ channels in guard cells were initially char acterized as sufficient but not rate limiting for stomatal opening. It was stated that "residual K+ currents measured after reduction by 10 mM Ba2+ (90% block) were estimated still to be suffi cient to allow stomatal movements in physiologically observed periods" (Schroeder et a!., 1987 p. 4112). Recent data have shown that the rate of stomatal opening is only decreased when inward K+ channels are blocked by 90% (Kelly et a!., 1995). Therefore, stomatal opening is likely to be controlled by the rate-limiting plasma membrane H+ pumps which are tightly regulated in guard cells (Kinoshita et a!., 1995). Stomatal closing is driven by the reduction in guard cell turgor, which requires the efflux of large amounts of K+ and anions and a parallel conversion of malate to starch (Raschke, 1979; MacRobbie, 1981; Outlaw, 1983). Outward-rectifying K+ channels were identified and character ized as a pathway for K+ efflux during stomatal closing (Schroeder et al., 1984; Schroeder et a!., 1987; Schroeder, 1988; Blatt, 1990; Blatt and Armstrong, 1993). Detailed studies have shown that K+ efflux can be mediated by outward-rectifying K+ channels in the plasma membrane that are activated by membrane depolarization (Fig. 1) (Schroeder et aI., 1987; Schroeder, 1988; Blatt and Armstrong, 1993). ABA enhances outward K+ channel currents (Blatt, 1990) via an ABA-
