SEASONAL BIRD USE OF CANOPY GAPS IN A BOTTOMLAND FOREST PDF
Preview SEASONAL BIRD USE OF CANOPY GAPS IN A BOTTOMLAND FOREST
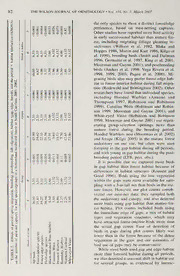
The Wilson Journal ofOrnithology 19(l):77-88, 2007 1 SEASONAL BIRD USE OE CANOPY GAPS IN A BOTTOMLAND FOREST LIESSA T BOWEN,' CHRISTOPHER E. MOORMAN,'-^ AND JOHN C. KILGO^ — ABSTRACT. Bird use of small canopy gaps within mature forests has not been well studied, particularly across multiple seasons. We investigated seasonal differences in bird use of gap and forest habitat within a bottomland hardwood forest in the Upper Coastal Plain of South Carolina. Gaps were 0.13- to 0.5-ha, 7- to 8- year-old group-selection timber harvest openings. Our study occurred during four bird-u.se periods (spring mi- gration, breeding, postbreeding, and fall migration) in 2001 and 2002. We used plot counts and mist netting to estimate bird abundance in canopy gaps and surrounding mature forest habitats. Using both survey methods, we observed more birds, including forest-interior species, forest-edge species, field-edge species, and several indi- vidual species in canopy gap and gap-edge habitats than in surrounding mature forest during all periods. Inter- actions between period and habitat type often were significant in models, suggesting a seasonal shift in habitat use. Bird activity generally shifted between the interior ofcanopy gaps and the immediate gap edge, but many species increased their use of forested habitat during the breeding period. This suggests that many species of birds selectivelychoosegap andgap-edge habitatoversurrounding matureforestduringthenon-breedingperiod. Creation ofsmall canopy gaps within a mature forest may increase local bird species richness. The reasons for increased bird activity in gaps remain unclear. Received 8August 2005. Accepted 12 July 2006. Many species of birds, including several Moorman and Guynn 2001). Forest canopy species of conservation concern that breed in gaps may be used differently throughout the mature forests, require some amount of forest year, depending on the availability of protec- disturbance to create ideal habitat (Hunter et tive cover, desirable nesting habitat, or suit- al. 2001). One type ofdisturbance common in able prey items (Robinson and Holmes 1982, mature forests occurs when trees fall from fire, Willson et al. 1982, Blake and Hoppes 1986). ice, wind, or insect damage creating small During migration, birds pass through unfa- light gaps in the forest canopy. Such gaps pro- miliar habitats and tend not to spend much vide microclimates and habitat patches that time in any one location (Moore et al. 1993). lead to a unique assortment of gap-associated Habitat selection during these periods may be flora and fauna (Watt 1947, Canham et al. influenced by available food resources, com- 1990), and increase the heterogeneity of veg- petition with other species, and risk of pre- etation structure in the forest. Canopy gaps dation (Petit 2000). During the breeding pe- created by small-scale timber harvest opera- riod, birds require habitat with suitable nesting tions may mimic these natural disturbances. sites. Birds that breed in early successional Birds select habitat based largely upon veg- habitats, including Common Yellowthroat and etation structure (Holmes et al. 1979), and Indigo Bunting (scientific names in Appen- some may prefer early successional gap hab- dix), use regenerating canopy gaps for nesting itat based on the unique qualities of the veg- (Moorman and Guynn 2001). During the post- etation (e.g., dense foliage, well-developed breeding period, adults may select densely herb and shrub layer). Several bird species vegetated habitats as refugia while molting seem to prefer small-scale canopy gap open- ings to mature forested habitat during migra- (Vega Rivera et al. 1999), and young may seek the protective cover from predators of- tion or the breeding period (Martin and Karr 1986, Germaine et al. 1997, Kilgo et al. 1999, fered by gaps (Anders et al. 1998, Vega Ri- vera et al. 1998), as each group is particularly ' Department of Forestry and Environmental Re- vulnerable at that time. sources, Campus Box 8003, North Carolina State Uni- Seasonal variation in the use of artificial, versity, Raleigh, NC 27695, USA. small-scale disturbances by birds within ma- 2Southern Research Station, USDA Forest Service- ture forests has not been well studied, and no Savannah River, R O. Box 700, New Ellenton, SC 29809, USA. research has systematically addressed the rel- ^Corresponding author; e-mail: ative use of gap habitat throughout the grow- [email protected] ing season, beginning with spring migration 77 78 THE WILSON JOURNAL OF ORNITHOLOGY • VoL 119, No. 1, March 2007 and ending with fall migration. Our goal was through 18 Oct). These beginning and ending to examine relative use ofgap and forest hab- dates are estimates ofbiologically meaningful itat by birds through four periods (spring, periods, but each overlaps extensively with breeding, postbreeding, and fall) within a bot- the other. Although many individuals initiated tomland hardwood forest to provide a more breeding on our study area before 16 May, comprehensive assessment of the response of transient species that breed to the north con- forest birds to canopy gaps. We hypothesized tinued to migrate through South Carolina until that relative bird use ofgaps would be highest mid-May. Similarly, some individuals migrat- during the non-breeding period when dense ed from or through our study area before 1 vegetative cover is important to dispersing September, but the bulk of fall migration oc- and migrating individuals. curred after 1 September. Plot counts were conducted within each of METHODS the 12 experimental gaps and within 12 for- — Study Area. We studied birds during 2001 ested control plots of equivalent size. The 12 and 2002 at the Savannah River Site (33° 09' forested control plots were randomly placed a N, 81°40' W), a 78,000-ha National Environ- minimum of 100 m from the nearest gap cen- mental Research Park owned and operated by ter within the mature forest surrounding the the U.S. Department ofEnergy. Our study site study gaps. The forest plot perimeters were was a mature stand of bottomland hardwoods flagged so that observers could easily identify approximately 120 ha in size in Barnwell plot boundaries. Each of the 24 plots was vis- County in the Upper Coastal Plain of South ited three times during each period and counts Carolina. We surveyed birds in 12 group-se- were averaged over the three visits. For ap- lection gaps harvested in December 1994 and proximately one half of the plot counts and in the mature forest adjacent to gaps. Gaps equally distributed across treatment types, two were of three sizes (0.13, 0.26, and 0.50 ha) observers walked slowly around the perimeter with four replicates of each size. It is within of each plot, recording all birds seen and this size range that previous research has iden- heard. When the observers met on the oppo- tified a threshold in response by breeding site side of the plot, they compared observa- (Moorman and Guynn 2001) and fall migrant tions and agreed upon a total number for each birds (Kilgo et al. 1999). The mature forest bird species observed within the gap-edge canopy was dominated by laurel oak (Quercus habitat. When only one observer was avail- laurifolia), cherrybark oak (Q.falcata var. pa- able, the single observer walked slowly godaefolia), sweetgum {Licjuidamhar styraci- around the entire plot. At both forest and gap fiua), and loblolly pine (Pinus taeda). The plots, birds observed within the actual plot midstory was poorly developed, consisting and at the immediate edge (0-10 m from the primarily of red mulberry {Morns rubra), bole line or flagged boundary into the forest) ironwood (Carpinus caroliniami.s), and Amer- were included in the count. Surveys varied ican holly {Ile.x opaca). The understory was widely in length (15 to 45 min); larger plots dominated by dwarf palmetto {Sahal minor) and plots with more bird activity took longer and switchcane {Arundinaria ^igantea). Veg- to survey. The percentage of gap habitat in etation in the gaps was approximately 1-8 m plot counts increased as gap size increased. in height and was dominated by regenerating However, the effect of gap size on bird use trees (primarily sweetgum, loblolly pine, syc- was not significant {P > 0.05) and we did not amore [Platanus occidentalis], green ash include the variable in our models. [Fra.xinus peunsylranica], oaks, and black At each of the 12 study gaps, we placed willow [Sali.x nigra]), and dense stands of three constant effort mist-net stations along a blackberry {Ruhus spp.), dwarf palmetto, and line emanating southward from the gap center: switchcane. — one at the approximate gap center, one at the Bi?d Surveys. We surveyed birds each gap edge perpendicular to and bisecting the m year during four avian activity periods: spring tree line, and one 50 into the surrounding migration (25 Mar through 15 May), breeding forest. The interior gap mist net was a proxy (16 May through 30 Jun), postbreeding (1 Jul for gap abundance, the gap-edge net was a through 31 Aug), and fall migration (1 Sep proxy for edge abundance, and the 50-m-into- Bowen el al. • SEASONAL BIRD USE OE CANOPY GAPS 79 the-forest net was a proxy for forest abun- hrs as the dependent variable for mist-netting dance. During the spring migration, post- analyses. For plot count data analysis, habitats breeding, and fall migration periods, netting included gap-edge and forest; for mist-netting was conducted once each week at each gap, data analysis, habitats included gap, edge, and rotating among gaps on a regular weekly forest. We considered habitat type and period schedule. During the breeding period, nets as fixed effects, with habitat type as a split were operated once every 2 weeks because plot factor and period as the repeated measure. birds tend to remain fairly stationary during We used the test for the habitat X period in- this period. Nets were opened at first light and teraction to assess whether habitat use was operated for 4-6 hrs, depending on daily consistent across periods (i.e., an interaction weather conditions. Netting was not conduct- between the two variables indicated that rel- ed when wind exceeded 16 km/hr or during ative use of the habitats differed among the steady rainfall. Nets were 12 m long X 3 m periods). Significant interactions generally tall, with 30-mm mesh. Captured birds were were the result of varying extents of differ- classified to age and gender (Pyle 1997), ences among gap, edge, and forest use but in weighed, and banded with a federal aluminum a consistent direction across periods. We in- leg band. We operated mist nets for a total of teipreted period and habitat effects separately 7,669 net hrs over the 2 years of the study. even when there was an interaction between Mist-net surveys and plot counts were not the two variables. Years were not significant meant to be directly comparable, but rather {P > 0.05) in any model and were pooled in separate, distinct measures of bird use of gap the final analyses. These pooled data are rep- and adjacent forest habitat in each of four resented in tables and figures. bird-use periods. Plot counts at gap sites in- We assigned birds to habitat-use groups cluded both gap and edge habitat, so the per- (Appendix): (1) all birds, (2) forest-interior centage of bird use of gap per se versus the species, (3) forest-edge species, and (4) field- first 10 m of forest (i.e., the edge) could not edge species (Ehrlich et al. 1988, Hamel be measured seasonally as it could for mist- 1992). We analyzed mist-netting captures and net captures. We chose not to note whether plot count detections for each group. Individ- birds specifically were recorded in the 10-m ual species were chosen for analysis if they outer band of gap and control plot counts be- accounted for at least 80 detections over both cause birds often moved back and forth across years for plot counts (Blue-gray Gnatcatcher, the boundary as they foraged. Additionally, Carolina Wren, Tufted Titmouse, Northern we were most interested in bird use of gap- Cardinal, Northern Parula, and White-eyed edge habitat compared to an equal size area Vireo) or at least 80 captures over both years of mature forest. Finally, forest mist-net sta- for mist netting (Black-throated Blue Warbler, tions were not placed with control plot count Carolina Wren, Hooded Warbler, Kentucky circles because the best location (i.e., at least Warbler, Northern Cardinal, and White-eyed 100 m from the nearest gap center) for plot Vireo). We included species that bred at our counts frequently did not lie along the south- study site and transient migrants that bred to ward emanating mist-net transect. Mist nets the north in our analyses. Birds considered and plot counts each have their limitations, winter residents, present only in early spring but the combined use of the two sampling or late fall, were not included. techniques allowed us to more comprehen- RESULTS sively measure bird use of the gaps and ad- — jacent mature forest. — Plot Counts. From April through October Statistical Analyses. We used a linear in 2001 and 2002, we counted 1,711 individ- mixed model (PROC MIXED, SAS Institute, uals representing 70 species in gap-edge hab- Inc. 1990) to perform repeated measures AN- itat and 38 species in forest habitat. We de- OVA comparing the effects of habitat type, tected more individuals in the gaps than in the period, and the interaction between habitat surrounding forest during all periods for all and period on bird abundance. We used mean bird groups and individual species analyzed birds per ha as the dependent variable for plot (Table 1, Fig. 1). The abundance of forest- count analyses and mean captures per 100 net interior birds, field-edge birds. Blue-gray 80 THE WILSON JOURNAL OF ORNITHOLOGY • Vol. 119, No. 1, March 2007 TABLE 1. Effects ofperiod (spring migration, breeding, postbreeding, fall migration), habitat (gap-edgeand forest), and the period X habitat interaction (ANOVA) on abundance of bird species/groups detected on plot counts ofgaps and forest areas in a bottomland hardwood forest in South Carolina, 2001-2002. Period Habitat Period X habitat Speciesorgroup F df p F df p F df p All birds 1.00 3,162 0.40 49.71 1,22 <0.001 0.66 3,162 0.58 Forest interior species 4.94 3,162 0.003 24.05 1,22 <0.001 0.83 3,162 0.48 Forest-edge species 2.10 3,162 0.10 60.16 1,22 <0.001 0.50 3,162 0.68 Field-edge species 27.55 3,162 <0.001 85.05 1,22 <0.001 27.90 3,162 <0.001 Blue-gray Gnatcatcher 14.08 3,162 <0.001 42.82 1,22 <0.001 5.80 3,162 0.001 Carolina Wren 9.44 3,162 <0.001 83.17 1,22 <0.001 1.76 3,162 0.16 Tufted Titmouse 12.78 3,162 <0.001 18.70 1,22 <0.001 2.22 3,162 0.088 Northern Cardinal 4.60 3,162 0.004 32.76 1,22 <0.001 0.60 3,162 0.61 Northern Parula 9.63 3,162 <0.001 19.43 1,22 <0.001 2.65 3,162 0.052 White-eyed Vireo 1.82 3,162 0.15 30.56 1,22 <0.001 1.49 3,162 0.22 Gnatcatcher, Carolina Wren, Tufted Titmouse, groups being most frequently captured during Northern Cardinal, and Northern Parula dif- spring migration (Table 2, Fig. 2). fered among periods, but no consistent pat- There was an interaction between period terns were evident, as seasonal use varied con- and habitat type, indicating a seasonal shift in siderably by species or group (Table 1, Fig. habitat use, for all birds, forest-interior birds, 1). forest-edge birds, field-edge birds. Black- Interactions between period and habitat throated Blue Warbler, Carolina Wren, Hood- type existed for held-edge birds. Blue-gray ed Warbler, Kentucky Warbler, and White- Gnatcatcher, and Northern Parula (Table 1 ). eyed Vireo (Table 2). Some species (e.g., for- Field-edge birds were detected most often est-interior specialists and Kentucky Warbler) during spring and fall migration and primarily shifted from gap during spring migration to in gap-edge habitat (Fig. 1 ). The greatest pro- edge during the breeding period and back to portion of forest detections of field-edge birds gap habitat after the breeding period (Fig. 3). occurred during the postbreeding period. The Forest-edge birds were most abundant in the Blue-gray Gnatcatcher was most abundant in gap habitat during spring and fall migration, gap-edge habitat during all periods, but forest but both gap and edge were used equally dur- detections decreased to almost zero during fall ing the breeding and postbreeding periods. To- migration (Fig. 1). Northern Parula used both tal mist-net captures tended to shift slightly gap-edge and forest habitat during spring mi- between gap and edge habitat (gap during gration and the breeding period, but almost all spring and fall migratory periods, edge during detections were in gap-edge during the post- breeding and postbreeding), with forest cap- breeding period—and fall migration (Fig. 1). tures representing just a small proportion of Mist Netting From April through October captures during each period. The highest pro- . in 2001 and 2002, we captured 1,476 birds portion of forest captures, however, occurred representing 56 species. We captured 55 spe- during the breeding period (Fig. 3). Forest- cies in gap and edge habitat, and 26 species in interior birds, forest-edge birds, Carolina forest habitat across all periods. We captured Wren, and Hooded Warbler used forested hab- more individuals in the gaps and at theiredges itat most during the breeding period (Fig. 3). than in the surrounding forest during all pe- DISCUSSION riods for all bird groups and individual species except the Carolina Wren, which was captured We observed and captured more birds in more frequently at edge or forest habitats than gap and gap-edge habitat than in the surround- gaps during all periods (Table 2, Fig. 2). Num- ing mature forest during all bird-use periods. ber of captures differed among periods for all Generally, bird detections in edge habitat were groups and species analyzed except Kentucky more similar to detections in gap habitat than Warbler and Northern Cardinal, with most mature forest habitat. The Carolina Wren was Bowen el al. • SEASONAL BIRD USE OE CANOPY GAPS SI Gap-edge Forest A B c D E F Birds/ha G H 11 “1— ^ ^ 1i i J n — t- J- A jr ^ X- "T T , Spring Breed Post Fall Spring Breed Pdst Fall Period FIG. 1. Seasonal plot counts (mean birds/ha) for gap-edge (open bars) and forest habitats (filled bars), with standard error bars (2001 and 2002 in South Carolina). (A) all birds, (B) forest-interior species, (C) forest-edge species, (D) field-edge species, (E) Blue-gray Gnatcatcher, (F) CarolinaWren, (G)TuftedTitmouse, (H)Northern Cardinal, (I) Northern Parula, and (J) White-eyed Vireo. ' 82 THE WILSON JOURNAL OF ORNITHOLOGY • Vol. 119, No. 1, March 2007 the only species to show a distinct forest/edge (ANOVA) OodOodOodOcmdord'ordo-od-rOd(tdN-0doN preference, based on mist-netting captures. Other studies have reported more bird activity V V V V in early successional habitats than mature for- est, including migrating foliage gleaning in- interaction sectivores (Willson et al. 1982, Blake and r-j <ON—(N (N Hoppes 1986, Martin and Karr 1986, Kilgo et \0 d d d (N d d d d d al. 1999), breeding birds (Smith and Dallman habitat 1996, Germaine et al. 1997, King et al. 2001, X Moorman and Guynn 2001), and postbreeding oooorninoNONiT', ooo birds (Anders et al. 1998; Vega Rivera et al. period 1998, 1999, 2003; Pagen et al. 2000). Mi- grating birds also may prefer forest-edge hab- the itat to forest-interior habitat during fall migra- and 2001-2002. tion (Rodewald and Brittingham 2002). Other oooooooooo researchers have found that individual species, forest), VVVVVVVVVV including Hooded Warbler (Annand and Thompson 1997, Robinson and Robinson edge,Carolina, 1999), Carolina Wren (Robinson and Robin- n son 1999, Moorman and Guynn 2001), and (gap, m m ^ (r^ij rr^ii 0^ r—i r—o White-eyed Vireo (Robinson and Robinson South rm<^ m ri ri vroi ri ri' ro^i ri ri 1999, Moorman and Guynn 2001) use regen- habitat in erating group-selection openings more than mature forest during the breeding period. forest oOdnri^irior—or-Od^n'vtO Hooded Warblers nest (Moorman et al. 2002) migration), ^ O—n nri ri‘ — ON and forage (Kilgo 2005) in the mature forest hardwood understory on our site, but often were seen foraging in the gap habitat during all periods, fall OCOO_O _ _ and with young in gap habitat during the post- dOCdOdOdOd1/-JOd—OdOd——dCOiTiOdOrO) breeding period (LTB, pers. obs.). bottomland V V V V V It is possible that we captured more birds postbreeding, in gap habitat than forest habitat because of a differences in habitat structure (Remsen and in Good 1996). Birds using the low vegetation ri ri ri ri breeding, rdrrr rdrnr rdrrr rrdrr Cn—O rd—r rd—r rOOnNi dr—^i —drr wpliitnhginwitthhe gaa3p-smwtearllenmeotrtehaanvabiilradbsleinftohresmaam-- species/groups ture forest. However, our plot counts corrob- orated our mist-net data; they sampled both migration, rOdrNrOd-ordr'jdON-vO'OdocdsoCcrr-iirr,jidor, the understory and canopy, and also detected bird rr (N — rr 00 ri ri more birds using gap habitat than mature-for- for est habitat. Plot counts included birds using (spring the immediate edge of gaps, a mix of habitat types and vegetation structures, which may captures period have attracted forest-interior birds more than the actual gap center. Ease of detection of of mist-net birds in gaps during plot counts likely was Effects lower than in the forest because of the dense of do 03 vegetation in the gaps and our estimates of 2. OCl.-QOJ bird use of gaps may be conservative. number sOC—_ OotiJl)"O-dr- ^ I morWheitlheanmofsotrebsitreddshuasbeidtagtadpurainndgeadlglepehraiboidtsa,t TABLE the — CC-X— we also detected a seasonal shift in habitat use on for several groups, as evidenced by interac- Bowen et at. • SEASONAL BIRD USE OE CANOPY GAPS 83 H Edge Forest A B c hrs net Captures/100 Period EIG. 2. Mean bird captures/100 net hrs foreach habitat and period with standard errorbars (2001 and 2002 in South Carolina). (A) all birds, (B) forest-interior species, (C) forest-edge species, (D) field-edge species, (E) Black-throated Blue Warbler, (F) Carolina Wren, (G) Hooded Warbler, (H) Kentucky Warbler, (I) Northern Cardinal, and (J) White-eyed Vireo. 84 THE WILSON JOURNAL OL ORNITHOLOGY • Vol. 119, No. I, March 2007 —^Gap Edge -a- For FIG. 3. Percent of mist-net captures per period occurring in each habitat type (gap, edge, forest) in a bottomland forest (2001 and 2002 in South Carolina). (A) all birds, (B) forest-interior species, (C) forest-edge species, (D) field-edge species, (E) Black-throated Blue Warbler, (F) Carolina Wren, (G) Hooded Warbler, (H) Kentucky Warbler, (I) Northern Cardinal, and (J) White-eyed Vireo. lions between period and habitat; the relative spring and fall migration, while use of forest- proportions of gap, edge, and forest eaptures ed habitat tended to be greatest during the varied among periods. Generally, bird use of breeding period and lowest during the migra- gap and edge habitats was highest during tory periods. Other research has documented Bowen et at. • SHASONAL BIRD USB OF CANOPY (JAI\S 85 seasonal shifts in habitat use between the eas, particularly during periods of vulnerabil- breeding and postbreeding periods, particular- ity, such as during migration when birds move ly as fledgling birds moved from forested hab- through unfamiliar areas and during the post- itat into early- and mid-successional habitats fledging period when young are more vulner- (Anders et al. 1998; Vega Rivera et al. 1998, able to predators. Alternatively, birds could be 2()()3; Pagen et al. 2000), possibly in search tracking seasonal changes in the abundance of of greater cover or more abundant food re- arthropod food resources, if the relative abun- sources. Regenerating forest canopy gaps may dance of arthropods in gaps and forest habitat provide a necessary habitat type for birds dur- changes through the year. Additional work is ing seasons of increased mobility. needed to assess the relative importance of Gap interiors were not only densely vege- vegetation structure and arthropod abundance tated, but also contained early successional in affecting seasonal avian habitat use in fruiting species (e.g., winged sumac [Rhus co- southeastern forests. pcillina\ and blackberry), while other fruiting The creation of0.13- to 0.5-ha canopy gaps species such as poison ivy {Toxicodendron can increase habitat diversity within mature radicons) and hawthorn {Crataegus spp.) bottomland hardwood forest, thereby attract- were common at the immediate gap edge ing a greater number of foraging, breeding, (LTB, pers. obs.). We observed omnivorous and migrating birds. This practice may be par- birds eating fruits in gaps, including American ticularly beneficial in stands with a sparse un- beautyberry {Callicarpa americana), flower- derstory because of dense canopy closure, a ing dogwood {Cornus florida), grape {Vitis condition common to the mid-successional sp.), hawthorn, poison ivy, and winged sumac forests that dominate the southeastern United (LTB, pers. obs.). Fruit typically is most abun- States. Our gaps did not impact reproductive dant from late summer through early fall success of Hooded Warblers nesting in the (McCarty et al. 2002). Willson et al. (1982) surrounding forest (Moorman et al. 2002), reported that avian frugivores preferentially probably because of the extensive amount of visited natural forest openings during migra- forest cover in the landscape (i.e., the extent tory periods, even when these gaps provided of forest fragmentation is low). Further, Rob- no more fruit than surrounding forest habitat. inson and Robinson (1999) noted that long- We did not, however, find a corresponding term effects ofsmall-scale canopy gaps on the shift in habitat use for omnivorous species forest bird community are unlikely because such as Northern Cardinal, suggesting that the regenerating forest matures and returns to birds were meeting their nutritional needs pre-harvest conditions in a relatively short without closely following seasonal fruit avail- time. When the gaps we studied were 2-5 ability. years old (Kilgo et al. 1999, Moorman and Birds used regenerating canopy gaps more Guynn 2001 theircontrast with the surround- ), than mature forested habitat during all peri- ing forest, in terms of vegetation height and ods. Bird habitat use shifted slightly from structure, was dramatic. During the current gaps during spring migration to forest during study, the gaps were 7-8 years old and the the breeding period, then back to gaps during contrast was beginning to blur, with many the postbreeding period and fall migration. gaps more closely resembling the surrounding Reasons for these habitat selections and sea- forest than 3-year-old gaps; some saplings ex- m sonal shifts, however, remain speculative. It is ceeded 10 in height. possible that omnivorous birds use canopy Group-selection timber harvest could allow gaps more during periods of high fruit avail- generation of income concurrent with an in- ability, as canopy gaps are known for their crease in habitat diversity, especially in forests high fruit abundance (Levey 1990). However, where rates of natural canopy-gap creation fruit production within our canopy gaps was have been altered by prior human disturbance relatively low and highly seasonal, with no (e.g., fire suppression, even-aged timber har- fruit available during spring, one of the peri- vest, altered flooding regimes). Pashley and ods of highest bird use. We suspect birds may Barrow (1993) recommended a management select regenerating canopy gaps for the pro- regime that mimics natural disturbance to tection offered by these densely vegetated ar- maintain habitat heterogeneity. Our results — 86 THE WILSON JOURNAL OF ORNITHOLOGY • Vol. 119, No. I, March 2007 highlight the importance of this recommen- availability and foraging ofHooded Warblers in a dation, as birds used both forested and early bottomland hardwood forest. Condor 107:626- 635. successional habitat at different times during Kilgo, j. C., K. V. Miller, and W. P. Smith. 1999. the year. Effects of group-selection timber harvest in bot- tomland hardwoods on fall migrant birds. Journal ACKNOWLEDGMENTS of Field Ornithology 70:404-413. King, D. L, R. M. DeGraaf, and C. R. Griffin. 2001. Financial support for this project was provided by Productivity ofearly successional shrubland birds the USDACSREES National Research InitiativeCom- in clearcuts and groupcuts in an easterndeciduous petitive Grants Program (Award 00-35101-9307), forest. Journal of Wildlife Management 65:345- North Carolina State University, and the U.S. Forest 350. Service Southern Rese—arch Station. We thank the U.S. Levey, D. J. 1990. Habitat-dependent fruiting behav- Department ofEnergy Savannah Riverforproviding iour of an understory tree, Miconia centrodesma, access to the study site and the U.S. Forest Service and tropical treefall gaps as keystone habitats for Savannah River for logistical support, with special frugivores in CostaRica.Journal ofTropicalEcol- thanks to J. I. Blake and E. G. Olson. We thank R. A. ogy 6:409-420. Lancia, D. J. Robison, and T. R. Simons forreviewing Martin, T. E. and J. R. Karr. 1986. Patch utilization this manuscript. We are grateful for field assistance by migrating birds: resourceoriented?OrnisScan- from T. B. Champlin, S. M. Junker, K. M. Mack, and danavia 17:165-174. D. E. Westerman, and for statistical assistance from S. McCarty, J. P, D. J. Levey, C. H. Greenberg, and B. Donaghy, M. L. Gumpertz, and J. L. Sloan. S. Sargent. 2002. Spatial and temporal variation in fruit use by wildlife in a forested landscape. LITERATURE CITED Forest Ecology and Management 164:277-291. Moore, F. R., S. A. Gauthreaux, Jr., P. Kerlinger, Anders, A. D., J. Faaborg, and F R. Thompson, III. AND T. R. Simons. 1993. Stopover habitat: man- 1998. Postfiedging dispersal, habitat use, and agement implications and guidelines. Pages 58- home-range size ofjuvenile Wood Thrushes. Auk 69 in Status and management of neotropical mi- 1 15:349-358. gratory birds (D. Finch and P. Stangel, Editors). Annand, E. M. and F. R. Thompson, III. 1997. Forest General Technical Report RM-229. USDA, Forest bird respon.se to regeneration practices in central Service, Fort Collins, Colorado, USA. hardwood forests. Journal of Wildlife Manage- Moorman, C. E. and D. C. Guynn, Jr. 2001. Effects ment 61:159-171. of group-selection opening size on breeding bird Blake, J. G. and W. G. Hoppes. 1986. Influence of habitat use in a bottomland forest. Ecological Ap- resource abundance on use of tree-fall gaps by plications 1 1:1680-1691. birds in an isolated woodlot. Auk 103:328-340. Moorman, C. E., D. C. Guynn, Jr., and J. C. Kilgo. Canham, C. D., j. S. Denslow, W. J. Platt, J. R. 2002. Hooded Warblernesting success adjacentto Runkle, T. a. Spies, and P. S. White. 1990. Light group-selection and clearcut edges in a southeast- regimes beneathclo.sedcanopiesand tree-fallgaps ern bottomland forest. Condor 104:366-377. in temperate and tropical forests. Canadian Jour- Pagen, R. W.. F. R. Thomp.son, III, and D. E. Bur- nal of Forest Research 20:620-631. HANS. 2000. Breeding and post-breeding habitat Ehrlich, P. R., D. S. Dobkin, and D. Wheye. 1988. use by forest migrant songbirds in the Missouri The birder’s handbook: a field guide to the natural Ozarks. Condor 102:738-747. history of North American birds. Simon and Pashley, D. N. and W. C. Barrow. 1993. Effects of Schuster, New York, USA. land use practices on neotropical migratory birds Germaine, S. S., S. H. Vessey, and D. E.Capen. 1997. in bottomland hardwood forests. Pages 315-320 Effects of small forest openings on the breeding in Status and management of neotropical migra- bird community in a Vermont hardwood forest. tory birds (D. Finch and P. Stangel, Editors). Gen- Condor 99:708-718. eral Technical Report RM-229. USDA, Forest Hamel, P. B. 1992. Land manager’s guide to the birds Service, Fort Collins, Colorado, USA. of the South. The Nature Conservancy, Chapel Petit, D. R. 2000. Habitat use by landbirds along Ne- Hill, North Carolina, USA. arctic-neotropical migration routes: implications Holmes, R. T, R. E. Bonney, Jr., and S. W. Pacala. for conservation of stopover habitats. Studies in 1979. Guild structure of the Hubbard Brook bird Avian Biology 20:15-33. community: a multivariate approach. Ecology 60: Pyle, P. 1997. Identification guide to North American 512-520. passerines. Part I. Slate Creek Press, Bolinas,Cal- Hunter, W. C., D. A. Buehler, R. A. Canterbury, J. ifornia, USA. L. Confer, and P. B. Hamel. 2001. Conservation Remsen. Jr., j. V. and D. A. Good. 1996. Misuse of of disturbance-dependent birds in eastern North data from mist-net captures to assess relative America. Wildlife Society Bulletin 29:440-455. abundance in bird populations. Auk 1 13:381-398. KilCiO,j. C. 2005. Harvest-relatededgeeffectson prey Robinson, S. K. and R. T. Holmes. 1982. Foraging
