Sacoglossan Opisthobranchs on Northwestern Pacific Shores: Stiliger berghi Baba, 1937, and Elysia sp on Filamentous Red Algae PDF
Preview Sacoglossan Opisthobranchs on Northwestern Pacific Shores: Stiliger berghi Baba, 1937, and Elysia sp on Filamentous Red Algae
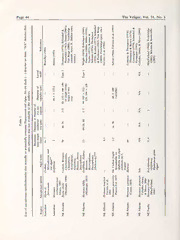
THE VELIGER TheVeliger 51(l):43-62 (March 31, 2010) <: CMS, Inc., 2008 Sacoglossan Opisthobranchs on Northwestern Pacific Shores: Stiliger berghi Baba, 1937, and Elysia sp. on Filamentous Red Algae CYNTHIA TROWBRIDGE* D. Oregon Institute of Marine Biology, University ofOregon, Charleston, Oregon, USA YOSHIAKI HIRANO J. Department ofBiology, Graduate School, Chiba University, Japan and Marine Biosystems Research Center, Faculty ofScience, Chiba University, Japan AND YAYOI M. HIRANO Marine Biosystems Research Center, Faculty ofScience, Chiba University, Japan Abstract. At least 20 species of sacoglossan opisthobranchs worldwide feed on delicately branching red algae; these species include members ofthree genera {Hermaea Loven, 1844; Stiliger Ehrenbergh, 1831; and Elysia Risso, 1818) in three sacoglossan families. The algal hosts include members of three algal families and at least ten algal genera in the order Ceramiales. We studied two sacoglossan species that feed on filamentous red algae: (1) the temperate to boreal Stiliger berghi Baba, 1937, on wave-sheltered shores ofHonshu and Hokkaido, Japan, and (2) the subtropical to tropical Elysia sp. on moderately wave-exposed shores of Okinawajima and Honshu, Japan. Preference experiments demonstrated that S. berghi prefers to associate with the alga Dasya when given pairwise algal choices but readily consumes members of several algal genera and exhibits no preferences between algal life- history phases (diploid tetrasporophytes vs. haploid female gametophytes). Elysia sp. is a small sacoglossan that consumes uniseriate and polysiphonous red algae. Given the small size and seasonally abundant populations of organismsthat feed on redalgae, wepredictthatthese sacoglossans and theirecological analogs on othershoresmay have an unexpectedly important role in consuming and/or fragmenting ceramialean red algae. Given the known propensity of these algae to be dispersed by international shipping and oyster mariculture, careful malacological consideration should be given to the potential ofsacoglossans to be inadvertent "hitchhikers" on a global scale. INTRODUCTION overlooked. Furthermore, surprisingly little malacolog- ical attention has been focused on the possibility that Marine red algae have been extensively introduced many ofthe newly reported Hermaea spp. may not be around the world by the fouling of ship hulls, the indigenous to the regions from which they have been transport and relaying ofoysters, and the introduction described. ofepiphytized host plants. Ofthemacroalgae currently The taxonomy and ecology of these specialized recognized as introduced (reviewed by Trowbridge, herbivores on native and introduced ceramialean hosts 2006), members of the red algal order Ceramiales in different areas of the world merits investigation. predominate. The dramatic proliferation of nonindig- Throughout temperate and tropical areas ofthe world, enous red algae (e.g., Womersleyella setacea covering there are numerous sacoglossan opisthobranchs that the benthos in the Mediterranean and "Heterosiphonia feed on species of Ceramiales; these sacoglossans japonicd" on Atlantic European shores) is widely belong to at least three sacoglossan families and three recognized among phycologists and invasion biologists genera: Hermaea Loven, 1844; Stiliger Ehrenbergh, but not among malacologists. The role of herbivory, particularly by sacoglossans, in controlling and/or 183O1u;ranodbjeEcltyisvieasRiosfsot,his18r1e8po(rTtabalree1)t.hreefold: (1) to exacerbating the spread of red algae has been long review andcomparethe sacoglossan opisthobranchs on ceramialean red algae worldwide, (2) to evaluate the *Mailingaddress: POBox 1995, Newport, OR97365 USA; similarities and differences ofthe algal hosts, and (3) to e-mail: [email protected] describe the reproductive features and feeding ecology <' 1 ' < Page 44 The Veliger, Vol. 51, No. 1 ^ O(— 0° 5^ =« O " m o O o ^<[> a O o c <D (U H Q 2 6 j2i a"s^ OC 3^ 2 " oc r< --—>uC—o1 O<^Gi3H^S vrO^coC&p£-NO)^ C(;sccaXc>^^-) oC"S=M«^—2c.cd.; _I •'Oi0-A^OM*^cNn0C)7O0Q-^i3c(3N0^i^U OHon 'c^/S^5 CCso-t O-—cCCc^NaO,HXu^t^3n-UOO:—^*ccNn.«a u ^H^O£S"^hm2aoCaSoO ocCdO £Po^; uo"i^r: 5T PoJ oiQ o c •73 m>u *^0) a < J03 JC3O H>i o T3 00 s 3 <N ^ >o -^ X (O—;, 'u < (OZl -Ut.- C1O) 'X' Wun)c^'«' >X— Xr)rt <Z sca C&O lr^»/-^li c« 0b.1U T>l 4- S c>cj 1 T3 -C 7 I s > H o e^ o I G a\I « c r>t o ^ ^ c sis < -V: ^(u o z O ^ ^'£ C O ca ? * •S ^^ 3 ^ 5 ^s'-:;^2 <?<i ".I^: ^«51) csru ,6, '<• a 63 ^3 t]q q $;<§ :^ a^ c 3 a < b c "cS o 6 O z CJ v^ O ^ 00 •S~^iNOO\ 1:1 tuc;S N®O S '-i ^ 3 $-c^ 3611 is : c3 aoc •S f' Sn~t3 ;:S<>:iu2 ^cCCuO CO «5JU cCOfl 3 ^cu § o ^~ =-S So c5u "^r! Co ^ "^ cu ^-^ COO a; oo O o a 3 CO I '3 O < < < <: < W 3 < Z ' 1 C. D. Trowbridge et al., 2008 Page 45 o o o OONN a G c c3 O X0u3 ^ a •^ 0a3 *-• =« E O~N eKnl X0^ u.5a2 M O <^acn^ ^aca .<K^^ X^>^,0^3 a1Cf)l. Xo aXo(ol<Vo^N>-Ja Ur(O".uyS"NN?,mO02rwg^3S-S0 ^Cr°OC_DO-0nCJ1<OcO0^ij UKH/Qct-as H/QU^ ^^>t1^J)i0Oc1icm<C-0Nyu1> U^^^lnm!]«> xOmlXi^^mmu/>nN^N X^*.3a3c)—. oX•OOZ>^00ciaInNN-^33aC^(X^jc-_N^^^X3^^c^21So"^^0-^\^3v CQ Ot2•O^rcN^^N^-«r .iO—^^^CcJ-nN^«3^OOr4NN1-—:NNnOO^! 25(0/)5 s LX>ogO3g!. £C>u«3 ^*;(1)1 Huf>t. H(f>UtN H-l Cfl ^ _; +(£-U•3tfrUt oI y rX«-i' n(~t « 03 03 Q ^ o ^ o U oj iu-l D. 3 X) S CS ft O 3 i« CC -^a -6^ ON ^ .„ "0^0 (O; QCHo3 T3cOjJ ^OC>^O ca. NNOO NXO, '03' J3 g 3 60 o id C y X a>,~s s ^ o N s ^ ^^ co S03 co •a c «^^^1Pt?iw 3o S- 3 s3 -ii^ eq 6 al C 03 a 5 ^ '5b u -2 ON 2Q (0N\ ^ 00 1 "^ aQ OiNn O2 OOSN « 5 ^O O'^N "??«2^? iao: iO'nn^ o ^ s§~ io™= id™= sS^o cg8? J8crt 25S 3o H; 03 t- t'isl ^S1; 000333 jj 03. ^S ^--•. -^C^) xff>l faj S^aS5 eou^n .•n8O"p3s^^§u=0^3 X1_Oa5p 13 c — X I'*3. &0H3 u .S- '(SU -c^ Onhi 03 =^ A "f>^t"*UfJt'Ua-5a'*n z Z z Z * * ^ * OD Page 46 The Veliger, Vol. 51, No. 1 oftwo Japanese species {StiligerberghiBaba, 1937, and Florida and the Gulf of Mexico is presently unclear, Elysia sp.) to enhance future identification of these as the radular tooth shape has never been reported; see NW species and to stimulate additional records within the discussion on Sea Slug Forum]. The Pacific S. North Pacific region. berghi was described from 15 specimens collected in 1935 from Tomioka, Amakusa, Japan (Baba, 1937); SACOGLOSSAN REVIEW the species was subsequently reported from Osaka Bay, Inland Sea of Seto, and Toyama Bay (Inaba, 1958, Within the genus Hermaea, the most abundant species 1962; Baba, 1959; Hamatani, 1961). This species has (in terms of population density) is Hennaea bifida rarely been reported, presumably owing to its small (Montagu, 1815); despite its frequency, the species' size, cryptic coloration, and patchy distribution. It has preference for native vs. introduced red algae on not been included in any of the recent popular books European shores has been overlooked. The uncommon on Japanese opisthobranchs (Suzuki, 2000; Nakano, to rare congeners include H. variopicta (Costa, 1869), 2004; Ono, 2004) or a recent Japanese sacoglossan which isconsidered by some authors to belongto genus account (Hamatani, 2000). The only literature report Hermaeopsis A. Costa, 1869; H. paucicirra Pruvot-Fol, for th—is species outside of Japan is from Russian 1953, on NE Atlantic shores; H. cruciata Gould, 1870, shores Peter the Great Bay, Sea ofJapan (Adrianov on NW Atlantic shores; H. oliviae (MacFarland, 1966) & Kussakin, 1998). Not only is there distributional and H. hillae Marcus & Marcus, 1967, on NE Pacific uncertainty but there is also taxonomic uncertainty. shores; H. wrangeliae (Ichikawa, 1993) on NW Pacific Baba & Hamatani (1970, p. 202) suggested that S. NW shores; H. nolo (Baba, 1959) on Pacificshores; and berghimay "belong to anothergenus, not yet defined." H. evelinemarcusae Jensen, 1993, on Australian shores. Finally, at least four Elysia spp. consume red algae [Note that Ichikawa reported her species as Aplysiopsis (Table 1). Elysia viridis (Montagu, 1804) on European wrangeliae, but that genus contains only green-algal shores feeds not only on four genera ofgreen algae but feeders, not red-algal ones; A. oliviae was moved into also on four genera of red algae, including native and Hennaea (Rudman, 2000, with a 2001 update; Behrens introduced species. The Australian Elysia cf. furva- & Hermosillo, 2005), based largely on diet. The first cauda consumes green algae in some months and author (CDT) considers A. wrangeliae to belong to the unidentified red algal epiphytes during other times genus Hennaea, though the absence of a voucher and (Brandley, 1984). On Japanese shores, Elysia abeil the lack of descriptions of radular teeth and the amakusana feeds on the red alga Griffithsia as well as reproductive systemhindersconfirmationofthegenus.] on green algae (Bryopsis, Cladophora, Chaetomorpha, Numerous additional Hermaea spp. have been record- etc.; Y.J. Hirano & Y.M. Hirano, unpublished ed: H. boucheti Cervera et al., 1991 (now considered to observations). Originally described as separate species, be H. bifida by Cervera et al., 2004), and H. ghanensis E. abei Baba, 1955, and E. amakusana Baba, 1955, are Caballeret al., 2006, on NE Atlantic shores; H. nautica currently presumed to be conspecific by many mala- NW Caballeret al., 2007, on Atlantic shores; H. coirala cologists (see www.seaslugforum.net). Finally, on Marcus, 1955, on SW Atlantic shores (considered to be Okinawan shores, there is a small Elysia sp. that feeds conspecific with H. cruciata by many colleagues); H. on uniseriate and polysiphonous filaments ofred algae. zosterae(Baba, 1959) onJapaneseshores(consideredto One specimen of this species has also been collected be conspecific with H. nolo by some colleagues; see from Shimoda, Izu Peninsula, Honshu. Our unde- Rudman, 2002); and congeners in the Indo-Pacific scribed Elysia sp. may be conspecific with Elysia sp. 2 region and Southern Ocean (e.g., Carlson&Hoff, 2003; from Hachijo-jima, Honshu (Nakano, 2004), Elysia sp. Bum, 2006; Sea Slug Forum, www.seaslugforum.net). 5 from the Ryukyu Islands (Ono, 2004), or an Elysia These species are often assumed to feed on red algae, sp. in the PhiHppines (T.M. Gosliner, personal although observational evidence is still frequently communication). Until the internal anatomy of these lacking. specimens has been characterized, we will limit our There are at least two Stiliger species that feed discussion to our Elysia sp. selectively on filamentous red algae: Stiligerfuscovitta- tus Lance, 1962, is an abundant, though insufficiently ALGAL HOST REVIEW studied, sacoglossan on NE Pacific shores (Lance, 1962; Case, 1972; Trowbridge, 2002) and S. berghi All ofthe sacoglossans that associate with red algae use Baba, 1937, on NW Pacific shores (Table 1). The NE hosts within the order Ceramiales; there are no Pacificspecimensof5.fuscovittatusbelongto thegenus confirmed reports of hosts from other algal orders Stiliger, not to Ercolania: the teeth are blade-shaped, (Table 2). Intriguingly, the hosts belong to three algal not sabot-shaped, and the species eats red algae, not families: the high-diversity Rhodomelaceae and Cera- green algae (characteristic o{Ercolania spp.). [The true miaceae as well as the comparatively lower-diversity identity of ""Ercolania fuscovitatta"" recorded from Dasyaceae (Table 2). As these families are distinguished C. D. Trowbridge et al., 2008 Page 47 Table 2 Classification of described hosts of sacoglossans that feed on red algae, based on currently recognized names in AlgaeBase (Guiryet al., 2006). The number ofalgal genera that are used as hosts are shown relative to thenumber of genera described in each algal family (based on Guiry et al., 2006). Orders Families Numberofgenera Genera References Ceramiales Rhodomelacae 1/137 (0.7%) Polysiphonia Baba (1937, 1959); MacFarland (1966); this report Dasyaceae 3/18 (16.7%) Dasya Van Bragt(2004); Lance(pers.comm. toCDT);Hansen(pers.comm. to CDT) Dasysiphonia Van Bragt (2004) Heterosiphonia Thompson (1976) Ceramiaceae 6/142 (4.2%) Ceramium this report Grijfithsia Robertson (1868) '; Trowbridge (unpubl. obs.); this report Halurus Wright (1859) -; Duerden (1896); Miller (1958)'; Kremer & Schmitz (1976)'; Trowbridge (unpubl. obs.)"* Bornetia Cornet & Marche-Marchad (1951) Wrangelia Ichikawa (1993) Callithamnion Lance (1962), Trowbridge (2002) 'G. corallina is considered to be G. corcdlinoides. -Q. setacea and 'G.jlosculosa are considered to be Halurusjlosculosus. primarily on reproductive attributes, what determines Marine Station, Hokkaido University; (2) the Misaki which families and genera within these families are Marine Biological Station (MMBS), on the Miura appropriate hosts for sacoglossans? All host genera are Peninsula, Honshu, University of Tokyo; and (3) eithermonosiphonous(withasinglerowofcellsattached Okinawajima, themainisland ofOkinawa. TheOshoro end-to-end) or polysiphonous (with a central series of Marine Station is on the southwestern side of cells surrounded by pericentral cells ofidentical height) Hokkaido on the Sea ofJapan (Figure lA); collections with large cells. Polysiphonous hosts are frequently were made at several sites within the wave-sheltered MMBS assigned to the genus Polysiphonia (Tables 1 and 2). bay. is on the Sagami Bay shores ofthe Miura However, given that the taxonomic status ofthe high- Peninsula on the Pacific coast of Honshu; collections diversitygenushasbeenhistoricallyinconstantfluxand were made from Araihama and Moroiso Bay at was recently subdivided into multiple polysiphonous MMBS (Figure IB). Finally, we collected sacoglossans clades (e.g., Womersleyella, Neosiphonia, Polysiphonia, from three sites (Zanpa-misaki, Sobe, and Sunabe) on etc.; seeChoietal., 2001), thenumberofpolysiphonous the southwestern coast ofOkinawajima (Figure IC). hostgenera recognizedbymalaco—logistsisundoubtedly an underestimate. Cortication the covering of the Collections and experiments: Our specific field collec- algal axis with a layer of small cells—occurs in a few tions were as follows. In May 2000, we collected host genera (e.g., Ceramium, Dasya), but most host Codiumfragile ssp. fragile (covered with epiphytes of genera lack cortication. Ceramium exhibits regions of the red alga Polysiphonia sp. and the sacoglossan cortication and regions without, thus providing saco- Stiliger berghi) from ropes suspended within Moroiso glossans access to small cells and large cells, respective- Bay at MMBS. We examined the single egg mass ly. Some genera, such as Dasya, have highly corticated produced by S. berghi in the laboratory (field-collected main axes but monosiphonous branchlets. What is not masses had already undergone several cell cleavages). clear, however, is why many red-algal genera, with Wemeasured the maximum and minimum length of 10 seemingly appropriate morphology for sacoglossan uncleaved ova and capsules within the egg mass. When feeding, are not reported as hosts. To what extent are veliger larvae hatched from the spawn mass, we the results in Table 2 a reflection of actual host use measured the maximum shell length of 10 veligers. versus an artifact of challenging phycological identifi- In July 2001, we collected >100 Stiliger berghi on cations by malacologists? We predict that future free-living red algae in various areas on the eastern collaboration between sacoglossan researchers and shore of Oshoro Bay, Hokkaido (Figure lA). We taxonomistsofredalgaewillrevealsubstantiallygreater conducted short-term feeding-preference experiments host diversity than is currently recognized. at the Oshoro Marine Station, Hokkaido University. We offered individual specimens of S. berghi pairwise METHODS choices ofthree algal hosts: Dasyasessilis, Polysiphonia sp., and Ceramium sp. We monitored the number of Study regions: The present investigation was conducted specimens on each alga periodically throughout a one- primarily at three regions in Japan: (1) the Oshoro day period and compared slug choices with Pearson's Page 48 The Veliger, Vol. 51, No. 1 Seaof Japan Jogashima d^p\ Figure 1. Map of three major study areas in Japan from north to south: (A) Oshoro Bay, Hokkaido; (B) Miura Peninsula, Honshu; and (C) Okinawajima, Okinawa. chi-square test and Fisher'sexact test on thecategorical In March 2004, we hatched veligers to remeasure data. shell length. Furthermore, we conducted another In spring 2004, 2005, and 2006, we collected feedingexperiment to determinewhether Stiligerberghi epiphytic and free-living Polysiphonia spp. from ropes exhibited feeding preferences for thalli ofdifferent life- suspended in Moroiso Bay. We also made collections history phases. Red algae belonging to Ceramiales o{Zosterafrom Moroiso Bay (with red algalepiphytes) typically have a complex life history with haploid and ceramialean red algae from the adjacent rocky female and male thalli (gametophytes) coexisting with shore, Araihama, at MMBS. morphologically similar diploid thalli (tetrasporo- C. D. Trowbridge et al., 2008 Page 49 phytes). Herbivores that feed on these algae may The species grew to 10 mm long and 11 mg in wet exhibit preferencesbetween the isomorphichaploid and weight, but most specimens were much smaller diploid phases. In March 2004, we conducted a (Figure 3A-C). Of 45 specimens examined in April pairwise-choice experiment with female gametophytes 2006 (Figure 3D), Stiligerberghihad 4 to 11 cerata per and tetrasporophytes of Polysiphonia harveyana. Prof. side (mean = 15 cerata total, maximum = 21); Chris Maggs (Queens University, Belfast) kindly however, some large specimens collected on 4 March identified the species on morphological and molecular 2004 had 15 cerata per side. grounds. The logistical details were similar to those of The species had pointed radular teeth with an the experiment above. In April 2008, we collected 8 unusual knob-like tip (Figure 4A-C). The ventral side specimens of S. berghi from Polysiphonia {sensu lata) ofeach tooth had a concavity, and the tip was blade- spp. and 1 from Ceramium sp. on the floating docks at like with small denticulations on the central, keel-like Hota on theeastern shoreofSagami Bay, Honshu. The edge. An extremely unusual feature was that the specimens were held in separate small containers filled denticulations were on both the ventral and the dorsal with seawater and offered the red alga Griffithsia edges of each tooth tip (Figure 4C). Based on 17 mm japonica. Observations weremade a fewtimes a day for specimens (1-3 long) collected in July 2001 from 6 days to evaluate whether the sacoglossan could Oshoro Bay, the number of ascending teeth varied consume Griffithsia. from 6 to 8; the number of descending teeth varied Finally, we made seven visits to Okinawajima from 17 to 23, includingthe three preradularteeth. The between July 2002 and April 2006. We collected length ofthe leading tooth varied from 80 to 135 |j.m; ceramialean red algae primarily at Sunabe, Chatan, the base width varied from 25 to 40 ^m. Our on the reef crest and at Zanpa-misaki on the reef observations of tooth shape, size, and number are platform. Smaller collections were also made in reef- consistent with the species description, with two rather crest and reef-lagoon areas of Sobe. Small, cryptic minorexceptions. (1) Baba(1937)mentionedthehighly sacoglossans could not be observed directly on the distinctive teeth were smooth, based on light micros- shore. Therefore, specimens ofElysia sp. were sampled copy; with currently available, high-resolution micros- by collecting red algae, holding them in shallow trays copy, the denticulation is readily apparent. (2) The with seawater for 1 to 2 days, and then hand-picking original description states there were five teeth in the the slugs from the algal surfaces or the air-water descending row and 19 teeth in the ascending row; we surface. Because ofthe physical conditions (moderately respectfully suggest that Baba inadvertently switched wave-exposedhabitats) and theecological ones (intense the terms ascending and descending. herbivory), the red-algal thalli were typically <1 cm long and could be identified only to genus. Reproductionanddevelopment: Stiligerberghicopulated SpecimensofElysiasp. wereheldinthe laboratoryin frequently in the laboratory. Slugs started by vigor- ously pressing the anterior part of the head together containers with different algal genera. Because of low sample size, formal experiments were not conducted, with that of a conspecific. The slugs then aligned but feeding damage on different algal genera was themselves right eye to right eye. Copulation took recorded when observed. The singleegg mass produced several minutes and was reciprocal; the short penis in the laboratory was collected; the diameters of ova lacked a stylet. The slugs copulated sequentially with and capsules were measured. many different slugs. The egg masses of S. berghi were oval and were RESULTS deposited among the branches of the polysiphonous red algae. The slightlyoval ova averaged 62 x 68 |im in Stiliger berghi diameter, and capsules were 104 X 136 |im. When veliger larvae hatched from the spawn mass in May External features: The simple rhinophores of Stiliger 2000, initial shell length averaged 116 |im. In March berghi had a distinctive brown stripe encircling the 2004, recently hatched veligers averaged 114 |im in upper end (Figure 2A-C). There was scattered purple- shell length (n = 5) and had type I shells (sensu brown pigment on the epidermis on the dorsal surface; Thompson, 1961). thesoleofthe footwaswhitealthough thepigmentwas present around the margin. This species had a very Feeding ecology: We found several algal hosts and pronounced C-shaped, brownish-purple esophageal observed feeding activities of Stiliger berghi. In May pouch (Figure 2B, C). The ingested red algal chloro- 2000, some ofthe subtidal thalli of Codiumfragile ssp. plasts were visible in the digestive tract, particularly in fragile that we collected from Moroiso Bay had the specimens that had recently fed. There were two epiphytic red alga Polysiphonia sp. We found 33 longitudinal tubes that extended branches into the individuals ofS. berghi on five thalli of C fragile with cerata but not into the tail. The anus was small and thisepiphyte. Cell size ofthe epiphyte on themain axes dorso-anteriorly situated. was about 200 \xm wide and 300-400 ^m long; on Page 50 The Veliger, Vol. 51, No. 1 Figure 2. (A) Oblique right view, (B) dorsal view, and (C) close-up ofhead ofa specimen ofStiliger berghi from Oshoro Bay, Hokkaido, Japan. Arrows indicatecharacteristiccolorbandson the rhinophoresand thevisuallydistinctesophageal pouch. (D-F) Dorsal and oblique views ofElysia sp. specimens from SW Okinawajima; (G) dorsal viewofElysiasp. from Shirahama, Shimoda, Honshu. Individuals ranged from 3 to 6 mm long. I C. D. Trowbridge et al., 2008 Page 51 20 n 12 n 10 •/ ^^^ Q 15 H O) . / / £, 8 • / .0) ^4-» / • J • i 10 O) 6 /•• H <u •o4- g > • / «!-» 4 y • May 2000 0) March 2004 Si 5 - . /, / E 3 2 - T" 5 10 15 Wet Weight (mg) Length (mm) B. D. 60 25 -1 5 20 - • • ••• •• •• • EZa Dasya • • • O •• ••• • Polysiphonia 15 • • • •• •• •• • •• • • •• • 5 10 July 2001 E April 2006 3 z 5 H 777771 12 -1— — 1 1 3 4 5 5 10 15 Wet Weight (mg) Length (mm) Figure 3. Body size ofStiliger berghifrom (A) Sagami Bay in May 2000, (B) Hokkaido in July 2001, and (C, D) Sagami Bay in March2004 and April 2006. terminal branches, cell size ranged from 200 |j.m wide Polysiphonia sp. (Figure 3B). Specimens onDasyawere and 340 jam long to small terminal cells 40 \xm wide not only more abundant but also significantly smaller and 40 |j.m long. Thus, a wide range of cell sizes was than those on Polysiphonia (Student's r-test, t = 4.7, available on which slugs could feed. P < 0.001). The overall sacoglossan abundance was In July 2001 at Oshoro Bay, Hokkaido, we found 0.86 slugs per gram (wet weight) ofceramialean algae. high densities of Stiliger berghi on polysiphonous red In feeding-preference experiments, 5". berghi preferred algae, primarily Dasya sessilis and secondarily on Dasya to Polysiphonia and strongly preferred both Page 52 The Veliger, Vol. 51, No. 1 mm Figure 4. (A-C) Radula ofa specimen ofStiligcr berg/ii from Oshoro Bay, Hokkaido, Japan. (D) Radula of2.5 Elysia sp. from SWOkinawajima. (E, F)Close-upofradularteethoftwospecimens. Scalebarsare(B) 50 ^m, (C) 20 |j.m, (E) 10 |im, and(F) 5 |im.
