Review Genetic heterogeneity of white-taüed deer: management lessons from a long-term study PDF
Preview Review Genetic heterogeneity of white-taüed deer: management lessons from a long-term study
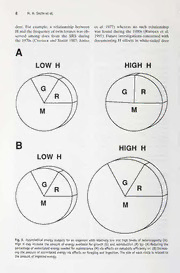
Mamm. biol. 66 (2001) 1-12 Mammalian Biology © Urban & FischerVerlag http://www.urbanfischer.de/journals/mammbiol ^Sß* Zeitschriftfür Säugetierkunde Review Genetic heterogeneity of white-taüed deer: management lessons from a long-term study By M. H. Smith, J. M. Novak, 3. D. Peles, and J. R. Purdue University of Georgia's Savannah River Ecology Laboratory, USA, Institute of Ecology, University of Georgia, Athens, GA, USA, DepartmentofGeneticsandSchoolofForestResources, UniversityofGeorgia, Athens, GA, USA, Ostermayer Laboratory, Pennsylvania State University, McKeesport, PA, USA, and ZooLogy Section, Illinois State Museum,Springfield, USA ReceiptofMs. 24.07. 2000 AcceptanceofMs. 20. 10. 2000 Abstract Genetic data from a long-term (16-year) study ofwhite-tailed deer (Odocoiteus virginianus) on the U.S. Department of Energy's Savannah River Site (SRS) were examined to evaluate spatialand tem- poral genetic heterogeneity in this species. Based on our analyses ofthe long-term data set, three major findings emerged, all ofwhich have important implications for management ofwhite-tailed deer: (1) There exists significant spatial genetic heterogeneity in white-tailed deer based on ana- lyses ofallozyme frequencies and mtDNA haplotypes. This heterogeneity exists on a much smaller spatial scale than would be expected for such a large and potentially mobile species as 0. virginia- nus. (2) The geneticstructure ofwhite-tailed deeratSRSistemporallydynamic and significant het- erogeneityexists within demographic units such as age and sex classes. (3) Levels ofgenetic Varia- tion, as measured by multilocus heterozygosity, are frequently correlated to characteristicsthatare important determinants ofecologicalfunction in white-tailed deer populations. These findings are evaluated in the contextofa generalmanagement modelfor0. virginianusthatis also applicableto other wildlife species. Key words: Odocoiteus virginianus, allozymes, mtDNA, spatio-temporal heterogeneity, demographic heterogeneity Introduction For most of this Century, population geneti- Wildlife biologistsconsideredchangesinpo- cists and evolutionary biologists have as- pulation numbers, quality of individuals sumed that populations consist of a large within them, and other demographic Para- number of randomly breeding individuals meters as being due to environmental ef- (panmixia). This view made it easier to fects, and genetic differences were often mathematically describe the behavior ofpo- not considered at all. Despite this, the envi- pulations and resulted in a relatively static ronmental or habitat model, which became concept of their genetic characteristics. Lit- the almost exclusive population dynamics tle effort was expended in linking genetic paradigm in wildlife biology, was very suc- and demographic changes in populations. cessful in explaining population differences. 1616-5047/01/66/01-001 $15.00/0. 2 M. H. Smith etal. The term "genetics" was not even men- examine existing genetic evidence to see tioned in most wildlife management texts howcommon spatial and temporal heteroge- during the first two thirds ofthis Century. neity is in white-tailed deer (Odocoileus vir- Technological advances in the 1950s and ginianus, Zimmermann). Our purpose is to 1960s made it much easier to describe char- review the literature on the genetics of the acter Variation among individuals and to white-tailed deer,present the results ofsome determine the genetic basis of this Varia- new analyses ofdata from a long-term study tion. There was a Virtual explosion in the of this species, and to propose a new per- number of studies that provided estimates spective on the important conceptual issues. of genetic Variation in natural vertebrate populations (Smith et al. 1982, 1994). As a result of these studies, it became clear that Sampling considerations the model of a large panmictic population was not correct for most terrestrial and Management decisions based upon data freshwater vertebrates (e.g. Smith et al. collected from public hunts need to be 1978; Avise 1994). However, most of the viewed with caution. Such data must be ex- data, especially for mammals, were from amined to determine if inferences can be small relatively short lived forms (e.g. expanded beyond the limits ofthe available Krebs et al. 1973). Data from the white- data in time and/or Space. Basically this re- tailed deer summarized here support the quires that animals are collected randomly view that genetic heterogeneity over short with respect to variables of interest such as distances maybe common eveninlarge,va- sex, age, antler morphology, genotype, etc. gile vertebrates. Deer collected on the Savannah River Site Temporal genetic heterogeneity over short (SRS) in the southeastern United States, time predicts the need for further refine- because of the limited public access and ment of habitat management models used the details of the hunting methods used, in wildlife management. Characteristics of can generally be considered to represent a concern to natural resource management, randomsample ofindividuals fromthe herd including conservation, need to be thought for most variables of interest (Novak et al. of as being due to the influences of Envi- 1991). Novak et al. (1991) found no hunter ronment (E; Habitat) + Genetics (G; Geno- selectivity based upon sex but some selec- type) + Environment-Genetic Interactions tivitybased upon age (older deerbeing pre- (E*G). A holistic perspective would dictate ferentially selected) thus slightlybiasingthe that the enviromnent-genetic interactions distribution of ages upwards. Thus age-re- would be at least as important in determin- lated genetic changes may be harder to de- ing the characteristics of wildlife species as tect than genetic changes related to sexual the main effects of genotype and environ- differences. ment. Studies that document differentialpo- pulation responses to similar environmental changes may indicate the importance of en- Spatial heterogeneity vironment-genetic interaction and/or differ- ences in the genetic composition of the ref- Many genetic studies have shown that erence populations. This Interpretation white-tailed deer populations are subdi- stresses the importance of genetic factors in vided spatially. The effect ismostnoticeable formulating management programs for both in analysesthat encompasslarge geographic game and nongame species. areas (Cronin 1989; Ellsworth 1994a, b; Genetics is most likely to be important if Hillestad 1984; Kennedy et al. 1987). In management units have different genetic these studies FSt (or a similar statistic that characteristics from each other and/or they estimates the proportion ofvariance among show temporal variations in their genetic populations) for both diploid (allozymes) DNA characteristics. Our primary objective is to and haploid (mitochondrial Genetic heterogeneityofwhite-tailed deer 3 [mtDNA]) genetic markers is large, indicat- (1991) found the FSTvalue for mtDNA to ing strong differentiation between local be9timesgreaterthantheFStforallozymes populations. inmuledeerfromMontanabutfoundnosig- On a small geographic scale, it is possible nificant difference between mtDNA and al- that spatial subdivision would not exist for a lozyme-derived FSxvalues for white-tailed large, potentially mobile mammal, such as deerfromMinnesota. the white-tailed deer. However, anumberof Generally, genetic differentiation ofpopula- studiesreject thisnotion. Spatial differentia- tions is attributed to reduced gene flow, his- tion ofpopulations for allozyme frequencies toric events and/or genetic drift (Cronin et was readily apparent in white-tailed deer al. 1991; Ellsworth et al. 1994a, b; Leberg from the Adirondack Mountains of New et al. 1994). In white-tailed deer, gene flow York (Mathews and Porter 1993), north- is influenced strongly by the species' mating eastern Minnesota (Cronin et al. 1991), and System, females being philopatric and males onanevensmallerscale,theSRS,SouthCar- doing the majority of movement among olina (Manlove et al. 1976; Ramsey et al. breeding groups (Nelson and Mech 1987). 1979), and Cumberland Island, Georgia The effect of extirpation in the late 1800s (Rowland 1989). When studied, mtDNA and subsequent restocking have had a pro- markersusually,butnotalwaysshowgreater foundeffect onthe spatialpatternofgenetic differentiation than those representing the differentiation of white-tailed deer popula- nuclear genome. For example, Cronin et al. tions over most of their ränge. However, in n ADA mm Allele | WebbWildlifeCenter Allele Q SRS mtDNA dt: Allele Haplotype Fig. 1. Comparisonofhaploid (mtDNA) anddiploid (allozyme) geneticmarkersforwhite-taileddeerpopulations collected in 1992 from the Savannah River Site (SRS; NmtDNA=215, NaLlozym=737) and Webb Wildlife Center (WEBB, NmtDNA=31, na[Lozym=32).Thepopulationsareseparated byapproximately100 km.Shownareaconitate hydratase (AH), adenosine deaminase (ADA), and L-iditol dehydrogenase (IDDH) (also known as sorbitol dehy- drogenase [SORDH]),thethreemostvariableofthe 13 locisampled. Designationsforallelesreferto relativemo- bilityinelectrophoreticstarch gels. Onlyhaplotypesandalleleswithfrequencies>0.01 areshown. 4 M. H. Smith etal. the coastal piain of South Carolina and force the idea that white-tailed deer are ge- Georgia, native herds were not hunted to netically subdivided on a finer geographic extinction and restocking was minimal. Re- scale than is apparent based upon their cent analyses of deer from SRS and Webb body size and vagility. Wildlife Center, located 100km apart on the coastal piain of South Carolina, docu- ment significant spatial heterogeneity in Demographic heterogeneity both nuclear and mtDNA genomes. Deer sampled from SRS and Webb center display Management decisions are usually made for markedly different genetic profiles for nu- a herd or larger grouping of individuals. clear and mitochondrial genes (Fig. 1). This However, smaller subsets of individuals and other studies (Kennedy et al. 1987) in- (age or sex classes) may be progressing dicate thatforallozymes all alleles at alocus along separate evolutionarytrajectoriessub- are present in most samples, although shifts ject to differing ecological challenges. These in frequencies are often observed. In con- demographic groups may exhibit different trast, mtDNA types, which are haploid and spatial or temporal patterns for both indivi- maternally inherited, are much more local- duals and genotypes. Thus, genetic variabil- ized. Sometimes, sampling locations sepa- ity must be analyzed with respect to demo- rated by only20km share no mtDNA types. graphic classes of age and/or sex within a Female white-tailed deer thus may be spatio-temporal context. The SRS deerherd extremely philopatric (Purdue et al. 2000). provides a unique opportunity to analyze The role offemale philopatryinthe mainte- such data because ofthe size ofthe data set nance of genetic structure of white-tailed within years (Minimum=409, Maxi- deer can be seen in an inadvertent "experi- mum = 1999, Total = 14221 deer), number ment" provided by the restocking of deer of years for which data are available (16) in Greene county on the piedmont ofGeor- and limited public access to the site. gia. Early in the twentieth Century, native Demographic heterogeneity in the SRS deer were extirpated from Greene and sur- deer herd was analyzed for the years 1974- rounding counties and never recolonized 1989 based upon 7Polymorphie loci avail- the area. In the late 1980s, extensive re- able in all years. Data for two highly Poly- stockingwas undertakeninthe area. North- morphie loci, ß-hemoglobin and transferrin, ern Greene county was supplied with were not available forthe year 1980, so that 60deer from Ossabaw Island and 7from year was not included in the analysis. Thus, adjacent Blackbeard island, Georgia all deer were categorized for multilocus (Blackard 1971). The Ossabaw Island deer heterozygosity class based upon 7loci carry a mtDNA type unique to the island (HCl was 0, 1, 2, 3 and 4+ heterozygous H and a few mainland localities on the lower loci, and [aresine ofsquare rootHC/Total coastal piain. In counties adjacent to number loci scored]), year of collection Greene, deer were transplanted from Texas (TIME), age class (AGE) (0.5, 1.5, 2.5, and Wisconsin. In 1994, the mtDNA of 3.5+ years), sex (SEX), and spatial unit 20 deer from Greene county were exam- (SPACE) (swamp or upland herd). Ex- ined. Seven of ten deer sampled in the panded definitions of the above variables northern part of the county carried the Os- can be found in Scribner et al. (1985) and sabaw island mtDNA type. The other three, Novak et al. (1991). plus 10 additionalindividualsfrom southern Probabilistic regression (PROBIT) analysis Greene county, displayed mtDNA types indicates that the distribution of AGE is a characteristic of deer from the Midwestern funetion of both TIME and SPACE United States. After 40years and 10- (X2 = 61.65, P< 0.0001 and x2= 13-09, 20generations, female deer from Ossabaw P = 0.0003, respectively). However, the dis- Island have apparently dispersed little be- tribution of SEX is a funetion ofTIME but yond their release site. These results rein- not SPACE (x2 = 48.24, P< 0.0001 and Genetic heterogeneityofwhite-tailed deer 5 X2=0.69, P= 0.4075, respectively). Thus, (F= 1.87, P= 0.0242), AGE and TIME analyses of genetic heterogeneity in rela- (F= 1.17, P= 0.2066), AGE and SPACE tion to AGE and SEX must be performed (F= 0.34, P=0.7930), and TIME and with the appropriate spatial and temporal SPACE (F= 1.64, P= 0.0621). No higher variables in the analysis. order interactions were significant, and Probabilistic regression using a Gompertz were therefore not included in the model. distribution for HC (GOMPIT) analysis in- The significant interaction of SEX and dicates that there are significant SPACE TIME is due to differenees in H between (X2=7.32, P= 0.0068) and TIME males and females in different years (X2 = 101.64, P<0.0001) effects, a marginal (Fig. 2). There is no consistent sexual bias AGE (x2 =6.59, P= 0.0863) effect and no in H, 6years showno significant difference, SEX (x2=0.02, P= 0.8989) effect. Unfortu- 5years show a male bias for higher H, and nately, interactions among dependent vari- 4years show a female bias (Fig. 2). ables cannotbe analyzedusing a probabilis- Previous analysis for the effects of age, sex, tic regression approach to account for year and spatial location on single locus TIME and/or SPACE heterogeneity of heterozygosity (h) for ß-hemoglobin by SEX and AGE. Therefore, an ANOVA Chesser et al. (1982) revealed slightly dif- was performed with H as the dependent ferent results. Sex was not found to be an variables and the main effect of SEX important variable although it is unclear (F=0.53, P= 0.4676), AGE (F= 0.82, whether a sex by year interaction was P= 0.4799), TIME (F= 3.84, P< 0.0001), tested. This analysis was performed over and SPACE (F=4.19, P=0.0406), and the only a three year time span, for only a Sin- two-way interactions of SEX and AGE gle locus and used simple tests of indepen- (F= l.ll, P= 0.3417), SEX and TIME dence that didnot analyze variables concur- 1973 1975 1977 1979 1982 1984 1986 1988 1990 1974 1976 1978 1981 1983 1985 1987 1989 Year Fig. 2. Multilocus heterozygosity values for male and female deer for the years 1974 through 1989. The year 1980isnotincludedasindicatedinthetext. 6 M. H. Smith etal. rently. As indicated by the analyses per- formation gathered to assess the additional formed here, there is a much larger ränge ecological and genetic dynamics that such of Variation in all variables when analyzed population substructuring introduces. over a longer time span. In addition, longer time series are more likely to include peri- ods of environmental stress. Thus, results Fitness correlates and energetics based upon data that are limited in time, Space or number of loci should be viewed Fitness correlates with caution. Differences in results can also be seen in the studies of Smith et al. (1990) Afitness correlate maybe defined as aphe- where a significant spatial effect was seen notypic characteristic in which the degree and Scribner et al. (1985) where a signifi- of expression is related to the survival and/ cant effect of Space was not seen. The first orreproductivesuccess(fitness)ofanindivi- study included data from a longer time se- dual. Numerous relationships between mul- ries (13years) than the second (6years) tilocus heterozygosity (H) andfitness corre- but both estimated H using the same seven lates have been demonstrated in a long- loci used here. term study of white-tailed deer on the SRS The above analyses illustrate the need to (reviewed by Rhodes and Smith 1992). examine demographic effects on genetic Within age classes ofmale deer, Hisrelated heterogeneityinlight ofspatial andtempor- to (a) body mass and fat levels (Scribner et alVariationofboth demographic andgenet- al. 1989), (b) antler size (Scribner et al. ic variables. Management decisions based 1989), (c) antler symmetry and Boone and upon only the main effect, SEX, would not Crocket scores (Smith et al. 1991), (d) fre- be the same as those based upon the inter- quency of spike antlers (Scribner et al. action of SEX and TIME. The interaction 1984), and (e) testicle size in fawns (Urb- of SEX and TIME is not surprising for the ston 1976). H in female deer is correlated SRS white-tailed deer herd given the rela- with(a) thefrequencyoftwinfetuses(Ches- tionships between male body mass and fat ser and Smith 1987; Johns et al. 1977), levels (Scribner et al. 1989), female fat lev- (b) age-specific body mass (Rhodes et al. els and their relationship to pregnancy 1991), (c) conception date and fetal growth (Cothran et al. 1987). conception date of rate (Cothran et al. 1983; Rhodes and females (Rhodes and Johns 1993) and fe- Johns 1993), and (d) bodyfatlevelspriorto male age specific body mass (Rhodes et al. conception and loss offat during pregnancy 1991). It is unclear if white-tailed deer are (Cothran et al. 1987). Fetal growth rate is unusual for mammals in how they partition alsorelatedtotheoverallHofthefetus(Co- genetic Variation in Space and time. thran et al. 1983; Leberget al. 1990). Although other studies have analyzed de- Smith and Risenhoover (1993) demon- H mographic heterogeneity, few have looked strated apositive associationbetween and at the interaction of age and/or sex with production of offspring in eight species of Space and none have analyzed differences cervids. In addition, relationships between over a comparable time span (Smith et al. H and fitness correlates have been observed 1994). The interaction of SEX and TIME in many other organisms (Allendorf and has direct consequences for the estimation Leary 1986;Mitton and Grant 1984). Thus, of genetically effective population sizes H likely integrates many important genetic andminimumviable population sizes. Ifdif- characteristics offorestorganisms. ferent demographic units are present in a The general trend ofthese relationships de- population andeachisprogressing alongin- scribed for white-tailed deer is for expres- dependent or semi-independent evolution- sion of the reference character to increase ary trajectories then management plans (e.g., antler size) or decrease (e.g., inci- needto encompass this heterogeneity. Man- dence of spiked antlers) with increasing agement decisions must be based upon in- number of heterozygous loci. However, the Genetic heterogeneityofwhite-tailed deer 7 functional relationship varies depending on er rate of protein turnover (Hawkins et al. both the specific character and the age of 1986). These findings suggest differences in the deer. In addition, there is evidence to maintenance metabolism amongindividuals suggest that expression of a reference char- with varying levels ofH. acter may decrease slightly at high H levels We hypothesize that increased energetic ef- compared to that of intermediate levels ficiencycouldexplainthe effectsofHonfit- (e.g., Chesser and Smith 1987) although ness-related characteristics in white-tailed this may be an artifact of small sample size deer. Hypothetical energybudgetsforanor- H at older age classes. ganismwithvarying are depictedinFig. 3. In most cases, H explains only a small per- In both homozygous and heterozygous indi- centage of the variability in characteristics. viduals, aportionofassimilatedenergymust For example, H is responsible for only 10- be utilizedformaintenance metabolism (M) 15% of the variability in main beam length which includes energy used for normal ac- and diameter of antlers, number of antler tivity. The remaining energy can be used points, and incidence of spiked antlers for secondary productivity (G + R). How- (Scribner and Smith 1990). Therefore, fac- ever, in the more heterozygous individual, tors such as age, body condition, habitat, increased energetic efficiency as a result of and resource quality, as well as their inter- higherHcouldreduce the amountofassimi- actionwith H, must be consideredwhen ex- lated energy required for maintenance me- A plaining the expression of fitness-related tabolism (M). slight decrease in the characteristics in individual deer. amount of energy needed for maintenance Although H may only account for a small could permit heterozygous individuals to amount ofthe variabilityin characters, deer partition much more energy for growth with high H generally grow faster, have and reproduction (G + R, Fig. 3a). higher body fat levels and higher reproduc- The above hypothesis assumes that ingested tive rates than deer with low H. These rela- energy (I) is relatively constant among indi- tionships suggest that deer with various le- viduals. However, individuals with higher H vels of H may partition their energy may be able to ingest more energy as a re- differently. The potential relationship of H sult of aggressive behavior (Garten 1976) to energetics requires furtherconsideration. or an increased scope of activity (Mitton and Grant 1984). Consequently, assimi- lated energy would be greater among more heterozygous individuals, providing more Heterozygosityand energetics energy for growth and reproduction, even An organism's energy budget can be de- if energetic efficiency is not affected by H scribed by I = A + E, where I is the total (Fig. 3b). ianmgoeustnetd,oAf eisnearsgsyimi(lKactaeld*engebrgoyd,yanmdasEs-1is) TtohereesfufletctionfaHsoenlecetnievregetaidcvsanistamgoestdluirkienlgy egested energy (egestion). Assimilated en- periods of stress (Koehn and Shumway ergy is partitioned into three categories 1982; Rodhouse and Gaffney 1984; Teska with A = M + G + R where M is mainte- et al. 1990). Teska et al. (1990) demon- nance energy and G + R represents assimi- strated that old-field mice ofvarying H dif- lated energy used for growth or reproduc- ferregardingfeedingefficiencyonly asfood tion (i.e., secondary productivity). quality is decreased. These results suggest A number of investigations have demon- that the effects of temporal Variation of H strated a relationship between H and ener- may be to decrease the ability to detect dif- getic parameters (reviewed by Mitton and ferences in H among individuals during Grant 1984). H has been correlated with non-stressful periods. decreased rate of oxygen consumption These findings may explain the inconsis- (Koehn and Shumway 1982; Mitton and tency of some relationships between H and Koehn 1985; Mitton et al. 1986) and a low- fitness correlates observed in white-tailed 8 M. H. Smith etal. deer. For example, a relationship between et al. 1977) whereas no such relationship H and the frequency oftwin fetuses was ob- was found during the 1980s (Rhodes et al. served among does from the SRS during 1991). Future investigations concerned with the 1970s (Chesser and Smith 1987; Johns documenting H effects in white-tailed deer A LOW H HIGH H Fig.3. Hypotheticalenergy budgets for an organism with relatively low and high Levels of heterozygosity (H). High H may increase the amount ofenergy available for growth (G) and reproduction (R) by: (A) Reducing the percentage ofassimilatedenergy neededformaintenance (M) via effects on metabolicefficiencyor: (B) Increas- ing the amountofassimilated energyvia effects on foraging and ingestion. The size ofeach circleis related to theamountofingestedenergy. Genetic heterogeneityofwhite-tailed deer 9 should take into account spatial and tem- boundaries of management units. In addi- poral Variation in environmental quality as tion, conservation efforts need to recognize well as in H. that many forms of a species having unique The influence of H on energetics is related combinationsofgenesmayoccurinsubpop- to individual fitness and quality of indivi- ulations separated by short distances. Spa- duals in a population. Genetic variability tial heterogeneity in gene frequencies has could be especially important in allowing been recognized in a wide diversity of ani- forest organisms to persist with increasing mals, anditsmanagementimplications have levels of anthropogenic and non-anthropo- been recognized as important in fisheries genic stress. Understanding the role of ge- management (Ryman and Utter 1987). netic Variation has important implications Wide scale fragmentation of forested habi- for both conservation and management tat can lead to reduction of census and ef- practices offorest wildlife species. fective population sizes, which may fall be- low the minimum viable size (Soule 1987). General management model One of the most important long-term ef- fects of falling below the minimum viable Genetic analyses ofwhite-tailed deer popu- population size is stochastic loss of genetic lations, as well as other animal populations, variability, which is important for both the have provided insights about their function- future evolution and the ecologieal func- ing that need to be incorporated in future tioning of populations. Small populations management plans (Smith et al. 1976). The may also be more susceptible to the effects results of these analyses are especially im- ofinbreeding, especially ifpopulation num- portant to the formulation of management bers are reduced quickly and kept low for plans.Theyare asfollows: 1) animalpopula- an extended period of time (Thornhill tions, especially white-tailed deer, show ge- 1993). Although we do not know whether neticheterogeneityoverrelativelyshortdis- genetic variability causes changes in popu- tances and amongdemographicunitswithin lation parameters and/or is a result ofthem, populations, 2) white-tailed deer popula- it would seem prudent to manage popula- tions, and probably those of other species, tions in a way that minimizes the chance of are generally dynamic over short time peri- losing genetic variability. ods, and 3) levels of genetic variability are The genetic strueture ofpopulations is tem- frequently correlated to many characteris- porally dynamic over time periods that in- tics that are important determinants ofeco- clude the length of typical studies (Smith logical functioning of populations and of et al. 1990). This dynamicbehaviorofpopu- concern to natural resource managers. lations may result from the interactions Althoughthecorrelationofgeneticvariabil- from smaller groups that differ from each ity and phenotypic characteristics do not other genetically. Animals that disperse usually explain a large proportion ofthe to- among these subpopulations to breed may tal Variation, eachcorrelation maybe some- have relatively outbred offspring with high- what independent such that the overall ef- er levels of genetic variability and different fects on the ecologieal dynamics of the phenotypic characteristics than those that populationfunction are veryimportant. breed within the subpopulation in which White-tailed deer show a surprising amount they were born. Management of forest ha- of spatial genetic heterogeneity even in bitats (e.g., maintaining corridors) to allow areas like the SRS where the habitats are this type ofdispersal among subpopulations not severely fragmented. In areas where may be essential to the long-term health of forested habitats are becoming even more manyofforest animals (Harris 1984), espe- fragmented (Harris 1984), spatial hetero- cially large vertebrates. geneity in gene frequency may be further One measure ofthe success ofvarious man- increased. Spatial genetic heterogeneity agement programs could be the degree to needs to be taken into account in defining which we maintain the genetic integrity of 10 M. H. Smith etal. the species. Genetic integrity must not be ciety continues to increase its impact on based on a static concept of the genetic everyhabitat on earth, itwillbe challenging characteristics of the species. Populations to devise management and conservation are extremely dynamic through Space and strategies for our precious life support Sys- time, andit seems prudent to manage biolo- tems, especially forests. gical resources so that they continue to ex- hibit their normal Variation in both Space and time (Norse et al. 1986). Thus, we are Acknowledgements trying to manage species that are likely to be genetically different in both Space and We wishtothankall ofthe people, andespecially time, and these genetic differences are PaulE.Johns, involved in collecting the electro- likely to have direct relationships with bio- phoretic data from the SRS herd. This research logical characteristics important to both wassupportedby contractDE-FC09-96SR18546 the survival of the species and the produc- between the University of Georgia and the tion of benefits for humans. As human So- U.S.DepartmentofEnergy. Zusammenfassung Genetische Heterogenitätbeim Weißwedelhirsch: Fürdie Wildbewirtschaftung relevante Erkenntnisse auseinerLangzeitstudie DatenauseinerLangzeitstudie (16Jahre) an Weißwedelhirschen (Odocoileusvirginionus) ausdem Sa- vannah RiverSite (SRS) des U.S.. Department of Energy wurden im Hinblick aufdas Vorkommen von räumlicher und zeitlicher genetischer Heterogenität bei dieser Art analysiert. Die Untersuchung er- brachte drei wesentliche Befunde, die auch für die Bewirtschaftung des Weißwedelhirsches von Be- deutung sind: (1) Wie aus der Analyse von Allozymfrequenzen und mtDNA-Haplotypen hervorging, besteht in Populationen des Weißwedelhirsches eine ausgeprägte räumliche genetische Heterogeni- tät, undzwaraufwesentlich geringerem Raum, als man dies beieinerpotentiellso mobilen Arterwar- tenwürde. (2) DiegenetischeStrukturderWeißwedelhirscheamSRSistzeitlichunterschiedlich undes gibteineausgeprägte Heterogenitätzwischen demographischen Entitäten wieAlters- und Geschlech- terklassen. (3) Dieinelektrophoretischen Untersuchungenermittelte Heterozygotierateisthäufig mit Merkmalen korreliert, diefürdie ökologischen Beziehungen in Weißwedelhirschbeständen bedeutsam sind. Diese Befunde wurden im Rahmen eines generellen Bewirtschaftungsmodells für 0. virginianus evaluiert, das auch fürandereWildtierarten anwendbarist. References Allendorf,F.W.;Leary,R.F. (1986): Heterozyg- Management of the Cervidae. Ed. by osityandfitnessinnaturalpopulationsofani- C.M.Wemmer. Washington, D.C.: Smithso- mals. In: Conservation Biology: The Science nianInstitutionPress.Pp. 168-177 ofScarcity and Diversity. Ed by M.E.Soule. Chesser,R.K.; Smith.M.H.; Johns,P.E.; Man- Sunderland, Massachusetts: Sinauer Associ- love,M.N.; Straney,D.O.; Baccus,R. ates.Pp.57-76. (1982): Spatial, temporal, and age-dependent Avise,J.C. (1994): Molecular Markers, Natural heterozygosity of beta-hemoglobin in white- History and Evolution. New York: Chapman taileddeer.J.Wildl.Manage.46,983-99. andHall. Cothran,E. G.; Chesser,R. K.; Smith,M.H. Blackard,J.J. (1971): Restoration of the white- (1983): Influences of genetic variability and tailed deerin the SoutheasternUnitedStates. maternal factors on fetal growth in white- M.S.thesis, LouisianaState University, Baton taileddeer.Evolution37,282-291. Rouge,Louisiana. Cothran,E.G.; Chesser,R.K.; Smith,M.H.; Chesser,R.K.;Smith,M.H. (1987):Relationship Johns,P.E. (1987):Fatlevelsinfemalewhite- of genetic Variation to growth and reproduc- tailed deer during the breeding season and tion in the white-tailed deer. In: Biology and pregnancy.J.Mammalogy68,111-118.
