Table Of ContentReaction of π Bonds
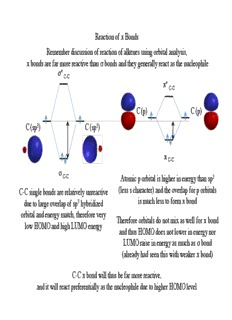
Remember discussion of reaction of alkenes using orbital analysis,
π bonds are far more reactive than σ bonds and they generally react as the nucleophile
σ*
C-C
π*
C-C
C (p)
C (p)
C (sp3)
C (sp3)
π
C-C
σ
C-C
Atomic p orbital is higher in energy than sp3
(less s character) and the overlap for p orbitals
C-C single bonds are relatively unreactive
due to large overlap of sp3 hybridized is much less to form π bond
orbital and energy match, therefore very
Therefore orbitals do not mix as well for π bond
low HOMO and high LUMO energy
and thus HOMO does not lower in energy nor
LUMO raise in energy as much as σ bond
(already had seen this with weaker π bond)
C-C π bond will thus be far more reactive,
and it will react preferentially as the nucleophile due to higher HOMO level
Electrophilic Addition to Alkenes
Alkenes generally react in an addition mechanism
(addition – two new species add to a molecule and none leave)
If hydrogen halides react, then a H and Cl add to the two ends of the double bond
!+ !- Cl
CH H Cl CH
3 3
H C H C
3 3
H
Since H-Cl is polarized, the H will be partially positively charged and Cl partially negative
The alkene is thus the nucleophile and the proton is the electrophile
The reaction is thus a two step reaction
The first step will generate a carbocation as a reactive intermediate
!+ !- Cl
Cl
CH H Cl CH CH
H C 3 H C 3 H C 3
3 3 3
H H
And the second step will have the carbocation react with the chloride to yield the product
(the chloride is the nucleophile and the carbocation is the electrophile)
Since the carbocation is the most unstable structure,
the first step is the rate determining step for this reaction
Regiochemistry of Alkene Additions
When E-2-butene was reacted with HCl, only one product can be obtained
!+ !- Cl
Cl
CH H Cl CH CH
H C 3 H C 3 H C 3
3 3 3
H H
When an unsymmetrical alkene, however, like propene is reacted,
two possible products are obtained (2-chloropropane or 1-chloropropane)
(resulting from H and Cl adding to different ends of alkene)
Cl
Cl
!+ !- H C H C
3 3
H Cl
H C
3
Cl
Cl
H C H C
3 3
Since the carbocation is the high energy structure along the reaction coordinate, the energy of
activation will be determined by the stability of the possible carbocations in the first step
Since 2˚ carbocations are more stable than 1˚, 2-chloropropane is the only product obtained
Regiochemistry of Alkene Additions
Hammond postulate is used to predict the relative rates of propene addition,
because the cation is the high energy structure along the reaction coordinate
the transition state for this reaction closely resembles the cation structure,
thus has a high amount of positive charge on the carbon
!-
Cl
!+ !- H
H Cl !+
H C H C
3 3
H C
3
H C
3
H C
3
Reaction Coordinate
Similar to a 2˚ cation is more stable than a 1˚ cation,
a partial positive charge on a 2˚ carbon is more stable than a partial positive charge on a 1˚ carbon
Regiochemistry of Alkene Additions
The hydrogen halide reactions with alkenes follow “Markovnikov” addition
Markovnikov addition – in an electrophilic addition, the heavier atom adds to the more
substituted carbon
The physical meaning behind the Markovnikov addition is the electrophile adds in such a
way to generate the most stable intermediate
What does this imply for a hydrogen halide reaction?
(remember that the first step is the creation of a carbocation)
Cl
Cl
Markovnikov product
!+ !- H C H C
3 3
H Cl
H C
3
Cl
Anti-Markovnikov
Cl
H C H C
3 3 product
Regiochemistry of Alkene Additions
When considering an addition to an alkene,
need to look at the two possible intermediate structures and compare their energies
(the more stable one will therefore react with a faster rate)
Some factors that could influence stability:
Resonance effects
We have observed previously that especially with charged species, structures that can
resonate the charge onto multiple atoms are more stable than compounds that isolate the
charge on a single atom
pKa
H
2
~16
C
H C O
3
O O
4.8
H C O H C O
3 3
Regiochemistry of Alkene Additions
The same effect can be observed in the carbocation intermediates for alkene additions
Cl
CH
3
H C
3
Cl
H Cl
or
Which is favored?
CH
3
H C
3
Cl Cl
CH CH
CH CH Cl 2 3
H C 3 H C 3 CH
3 3 3
The chlorine atom has lone pair of electrons
which can delocalize into empty p orbital of cation for resonance stabilization
The reaction of Z-2-butene with HCl thus only yields 2,2-dichlorobutane
Cl Cl
Cl Cl
H Cl
CH CH CH
3 3 3
H C H C H C
3 3 3
Regiochemistry of Alkene Additions
Any atom with lone pair of electrons adjacent to empty p orbital
can stabilize cation through resonance, realize though that resonance
can only occur with orbital alignment between adjacent atoms
OH
H+ H O
2
OCH OCH
3 3
OCH
3
OCH
3
The proton adds in the first step to generate the carbocation adjacent to the oxygen due to
resonance from the lone pair stabilizing the cation, thus directing the regiochemistry
Because the cation is more stable with resonance,
the relative rate compared to alkenes without the possibility of resonance is higher
Relative Rate (25˚C)
OH
H+/H O
2 5 x 1014
OCH OCH
3 3
H+/H O
2
1
OH
Regiochemistry of Alkene Additions
Resonance can also occur with extended p orbitals on adjacent carbon atoms
We observed the reaction of butadiene in discussing kinetic versus thermodynamic reactions
Isolated 1˚ cation
H+
or
!+
OH OH
2
!+
H O
2
2˚ cation in resonance
!+
OH OH
2
!+
Partial charge on 2˚ carbon More substituted double
more stable than 1˚ carbon, bond is more stable,
therefore top pathway is the therefore bottom pathway is
kinetic pathway
the thermodynamic pathway
In practice, kinetic pathway is favored at low temperatures
and thermodynamic pathway is favored at high temperatures
Effect of Hyperconjugation on Carbocation Stability
We have already observed that cations with more alkyl substituents are more stable
than cations with less alkyl substituents
CH H H H
3
H C CH H C CH H C H H H
3 3 3 3 3
Stability
The reason is due to a type of resonance with the neighboring C-H bond called
“hyperconjugation”
H
H
CH H H H
H C 3
2 H
CH H H H
3 H
The electrons in the neighboring C-H
The interaction is similar, but
bond stabilize the empty p orbital by
different, than the interaction between
donating electron density
two p orbitals in an alkene
This effect can only occur with a
(less interaction due to further
neighboring carbon,
distance in hyperconjugation)
therefore 3˚ > 2˚ > 1˚ > methyl cation
Description:inductive effects can alter carbocation stability. Inductive means “through . Some people have a nonfunctioning aldehyde dehydrogenase enzyme.