Table Of ContentTThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan
uetth T)'ans.Iepid.Soc.Jmpan 59 (2):87-102,Mar2c0h08
Physiologic sailde-effect model for diversificat oifo nnon-functional or neutral
trait sa: possible evolutionary history of lhnessa butterfiie (sLepidoptera,
Nymphalidae)
JojiM, OTAKI
Laboratory of Ce]l and Functional Biology, Department ofChemistry, Biology and Marine Science,
Faculty of Science, Universit yof the Ryukyus, 1 Senbaru, Nishihara ,Okinawa 903-0213, Japan;
e-mail:[email protected],ac.jp
Abstrac tButterf iwiyng color-patterns are highl ydivers eM.any color-patterns or their elements
are ecologically and behaviorall yfunctiona lin mating, mimicry and camoufiage, but it has been
thought that not all ef them have clearly identifiab lfeunction sH.ow such non-functional, some-
times extravagant color-pattern trait sevolved has been enigmatic. To illustr atthei sproces s,a
model for the celor-pattern evolution of the Admira] butterfii e(sth egenus Vtinessa senstt stricto) is
proposed. It is firs atssumed that ancestral pepulations of Vdnessa were geographica lilsyolate din
high altitude regions where temperatures fiuctuat ewidely. In thi smodel, pupae produce the cold-
shock hormone (CSH) to protec dtifferentia tceilnlgs in response to the temperature fiuctuation.
This horrnone has an opportunistic ability to modify the wing color-patterns as a side effect or
pleiotropi ceffect, revealing phenotypic plastici teyf the ancestral populations .The modified pheno-
types are rarely adaptive; they are in most cases either not favorabl ien mating or simp]y neutral
with no adverse or adaptive etfects, The modified phellotypc sare noneLheless canalized in a popu-
latio nthrough a process of genetic assimilation accornpanying natural selection for high hormonal
activity, resulting in diverse orange areas on the wings in the presen tVkeness aspecies livin gin high
altitude regions, Thus, the CSH physiologicall yacts as a mediator of geneti acssimilation of the
non-functional or neutral trait s,In other words, a non-funetional or neutral trait can be assimilated
in a population as a "parasite" of a functien atrlait .Followin gthe evolution of mating preferences,
prematin grepreductive isolatio nmight be established, as in the elassical mechanism for allopatric
speciation. This "physiological side-effect model" may explain the non-functional or neutral wing
color-pattern diversit yof Vttnessa and other butterfiies.
Key words Lepidoptera ,Nymphalidae, Vdnessa, color-pattern evolutien, phenotypic plasticit yg,e-
netic assimilation, pleiotrop sype,ciation, cold shock, non-functional trait ,neutra] trait.
Introduction
The variety of butterf iwiyng color-patterns functio nas visual signals for mates or preda-
tors, ar]d accordingly, many of them are importan ttrait fsor natural selection (Uesug 1i9,91;
Jiggin set al,, 2001; Kapan, 2001). In some species of butterfli essub,tle color-pattern
djfferenc ebsetween individual sare visually discriminated (Fordyc eet at,, 2002; Robertson
and Monteiro ,20eS) ,but in other species, only their rough pattern sb,ut not detailed pat-
terns, are thought to be functien aals matjng signals (Tinber gete anl,, 1942; Si]bergli eandd
[faylor ,1973; Hidaka andYamashita, 1975; RutowskL ,l977; Wiernasz ,1989).
Accordingly, it has been thought that not all of the wing color-pattems or their elements are
equally functiona lor adaptive, Some of the butterf lwiyng color-patterns or their elements
appear to be non-adaptive, and in certain cases, they could be simply extravagant. Vivid il-
lustration osf such cases can be found in seasonal polyphenism :it sadaptive significance is
only marginally known so fat r(Shapi r19o7,6; Nijhout, 1991) although it is possibl tehat un-
proven functio nmight simply indicat iencomplet eknowledge of the adptive significance. It
is the common view tha tthe color patter nof a given species is a product of historical
biological by factors,
chance and necessity woven natural selection and other
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan
88 JQjiM. OTAKI
From the viewpoint of developmenta lbiology ,the mechanism of the eyespot fbrmatio non
butterH ywings has been investigat eextdensively with surgical, physiological a,nd molecular
biologicatlechniques(Nijhou1t98,4,1991;French& Brakefield1,992,1995;Brakefiel&d
French, 1995; Brakefield et al,, 1996; McMillan et al., 2002; Monteiro et al., 2006). There
is much experimental data indicati ntgha tan eyespot focus on wings works as an organizing
center fbr the color-pattern development ,althoug hthe exact nature of the position aslignal
emerging from jt is still largel yenigmatic. The same organizing center seems to organize
the pupal wing cuticle spot (Ota keti al., 2005a) .In addition, the color pattern msay be de-
termined not only by the putativ peosition aslignal from organizing centers but also by the
cellular ability to interpr etthe positiona lsignal, which could be modified by more systemic
factor ssuch as ecdysteroids (Koc h& BUckmann, 1987; Koch et at,, 1996; Rountree &
Nijhout ,1995) and the putatiye cold-shock hormone (CSH) (Otak i1,998, 2003, 2007a;
Otaki& Yamamoto,2004a;Otaki 2005b).
et al,,
I have been intereste din the mechanism of wing-wide color-pattem determinati odnuring
development and it srelations to butterfl ycolor-pattem evolution and speciation, Here I dis-
cuss how non-functional or neutral color-pattern trait sevolved in butterfii e[sI,b i]Iustrate
the process ,I focus on the Admiral buttertli tehse, genus lexness aFabriciu s1,807 sensu
stricto, which is now composed of nine species in total (Walilb eetr agl., 2005; Otalc iet al.,
2006a;Vane-Wright& Hughes,2007).Molecular has that
phy]ogenetic analysis revealed
the genus liZines sseansu stricto can be divide dinto two groups: the indic agroup and the
atalanta group (Ota keti al., 2006a) (Fi gI.A). Furthermore ,we have previously pointed
out that one can arrange thes el,2ines sspaecies, but not closely-related species of genera
Ciynthi aFabricius ,1807 and Bassaris HUbner, 1821 (altho udgyhnth iaand Bassari sare no
longer formally valid as genera according to Wahlberg et al. (2005 )i)n ,a progressive linear
series of the colQr-pattern difference smainly based on the quantitativ evalue of "the relative
area of orange (RAO) "on the dorsal forewings (Ota k&i Yainamoto ,2004b) (Fi gI.B),
Intriguingly a, similar color-pattern series is known to be induced by systemic iajection sof
sodium tungstate into pupae of Iilanes isnadic a(Herbs 1t7,94) (Ota k&i Yarnamoto ,2004a)
(FigI.B).
The color-pattern phenocopies mentioned above are an experimental expression of pheno-
typi¢ plastici tthyat is usually hidden in V. indica ,Recent resurgent interes tin phenotypic
plastici tanyd ecological speciation in evolutionary biolog y(West-Eberh 1a9r8d9,, 2003;
Scheiner, 1993; Pigliucc i& Murren, 2003; Rundle & Nosil ,2005; Pigliucc iet al., 2006;
Price ,2006) could make the iinness agenus an importan tcase of plasticity-rel eavtoleu-d
tion ,Indeed, I have already shown tha tthe evolution of Linness kaershawi (McCoy ,1868) is
highly likel yto haye been triggered by a plasticity-relat meedchanism (Otak i20,07b),
Followin ga brie foverview of the color-pattern modifications of linness aI ,propose a possi-
ble scenario fbr the celor-pattern evolution of likeness bautterflie bs,ased on the fact that the
color pattern osf several Vtiness aspecies are similar to those of V, indic ainduced by cold
shock and other perturbatio n(sFig .1) .This "physio]ogica] side-effect model" explains not
only the color-pattern diversit yof iilrnes bsuatterfl ibeust alse of other butteifl itehast harbor
many non-adaptive, non-functional neurral trait s.Such non-functional and neutral traits
that accompany and even feature many species might have emerged similarly to thi smodel.
Although this model has not been proven yet, it is intende dto construct a theoretical frame-
work fbr futur eresearch, I also point out here that this model does not suggest an exclusive
means, and other mechanisms may have been responsible fbr the color-pattern diversi toyf
the genus Vkeness asensu stricto (Otak i2,008). Some ideas presente dhere were already
mentioned in Otaki et at, (2006b i)n Japanese.
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan
Side-EffecMtodelforNon-Functional Traits 89
The CSH its
putative and physiological eifect
lt has been known that the color patterns of butterf lwiyngs can be modified by the applica-
tion of cold shock to pupae immediatel yafter pupation (Nijho u1t98,4; Shapiro ,1984) ,The
pattern sare not randomly modified, but there is a clear and consistent tendency: many
color-pattern elements become obscure and some elements are erased or dislocate dI.n
lt2ines csanatui (Linnaeu 1s7,58) ,the b]ack spots on the forewings become smaller or weak-
er (Nijho u1t98,4; Shapiro ,1984) ,Although the response to the cold shock varies from in-
dividua tlo individua lth,e modified pattern scan be arranged from less severe to highly se-
vere in a linea rfashio n(Nljho u19t8,4) ,In contrast, a non-linear response was seen in heat-
shocked butterfii e(sOtak i2,007a, b). This is an importan tpoint to consider, because cold-
shocked-induced phenocopies b,ut not heat-shock-induced phenocopies a,re suggested to be
by
caused a single mechanism.
Is this type of modification mediated by a hormonal facto rthat is secreted in response to
cold shock? Alternative liys ,it the scale cells themselve sthat directl ryespond to cold shock
without any hormonal factor ?Tb resolve thi sproblem ,Otaki (1998 p)erformed hemolymph
transfer from cold-shocked to non-cold-shocked individual sa,nd fbund that the
modificatien-inducing propert ycan be transferred, although the induced modilications in
the recipients were relatively mild. The recipients showed dislocati oofn one of the color-
pattern elements, thc parafocal elements, toward the border ocelli (eyespot sre)du,ction of
black spots (henc eexpansion of the orange area), and other modifications as seen in cold-
shocked individua l(sOtak 1i9,98) .The existence of the putativ CeSH was then proposed in
the literatu rIet ,was also proposed that the CSH function sto change the threshold levels
for the putative morphogen without alfecting the morphogenic positiona linformatio intself
(Otaki1,998).
The chemical nature of the CSH has not yet been established, However, if a hermonal fac-
tor is eperating in the cold-shocked individual ist ,may be relatively easy to phenotypically
mimic the CSH effect by means of fbreig nchemicals includin gstmctural analogs that serve
as a ligan d(agon iosr tantagonist) for it sreceptor. The firs C`tcold-shock agonist" discov-
ered was sodium tungstate (Na,WO, )a ,supposed protein-tyros ipnheosphatas einhibitor
(Otak i1,998) .When ibjecte dinto pupae, this chemical and also related ones, sodium
molybdate (Na,MoO ,a)nd molybdic acid (H,MoO, )i,nduced pattern modifications that
were indistinguishabl efrom the cold-shock-treated one (Otak i19,98; Otaki & Yamamoto,
2004a) (Fig .IB). Tungstate and molybdate are likel yto be highl ystable in pupae because
of their simple chemical structures, This modification-inducing ability of tungstate is
shovJn to be largel yspecific to the color-pattern developrnen tan,d such modifications are
not observed with ibjection sof stress-inducing chemicals such as urea (Otal 1c9i9,8) ,thap-
sigargin, ionomycin, and geldanamycin (Otak eit at,, 2005b) .The pharmacologic aelffect
of tungstate is thus considered as a functiona mlimic of the putativ eCSH (Otak i1,998;
Otalc iet al., 2005b) . rlbtally diffk}r echnetmicals, a crude fungal extract (Umcbac h&i
Osanai ,2003) and hepari nand it srelated chondroitin sulfates (Serf a&s Carroll 2,005),
were then discovere dto exhibit a similar effect. AIso recently discovere dwere the "cold-
shock antagonists", which induce modifications in the "reverse directio n("Serf a&s Carroll,
2005; Otaki, 2007a) (Fig .IB). Each of these agonists and antagonists probabt yaffects a
distin catnd differe nptoint in the same molecular pathway for the development of the color
pattern osn the wings,
Although it is diracul tto establish the molecular identi toyf the CSH in these physiological
studies, these experiments revealed how and to what degree the butterfi ycolor-patterns are
plasti tco external perturbations .Importantl yth,e linea rarrangement of the modification-in-
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society oofJfap anJapan
90 JejiTVT,0T,xKI
A hE・f,B va n s gpa]odifi' ei'a ・ifndica
T.hl/livaiip,i'iirghiil-"iR ..
6 tsiStiV
100 .,Wti:
e
Bassarisgenerillit ceRtrattediorangeatta Eindica・ CExpe]dedergMgesUTet t"meait!eu
"
AJ]4LAIVIXCROUP
sPZ,ii81rnessa
eXatatanta
lhnessa
atalanta
38 Ar"XANm
GROUPlhnessatameamea
kctrNigts
a g
M,MC'A(:a"ollp
eg,,X,DIC.4ai{ov:}e72Usamani
hlh,rew:e
eeza
gguana
if defeant r' U buana K dilecta
eseex
Medifi
P2inessa ld
dojeanit' pmdvgepa.X..fonylj.・,
54
"
leovanessaseamani
ig・.",eti,,Ss.a
ptec:soQeesas-g
"
qnthia
-rex'""e
69
6S(;),nthiabraziliensis
hV
Cllntthia
cardui e
mpt>-MU<mpt
.lnneniawestermannl
--1- 1le changes
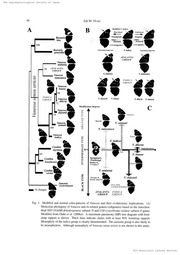
Fig. 1.eModified and norrnal color-patterns of Vinness aand their evolutionary implicatio ns(.A)
Molecula rphylogeny of lilaness aand its related genera (subgene rbaas)ed on the mitochon-
dria mlo5 (7VttD dHehydrogenase subunit 5) and COI (c.vtochr ooximdaese subunit D genes.
Modified frem Otaki et al. (2006a )A. maximum parsimony (MP) tree diagrarn with boot-
strap support i sshown. Thick lineg indicat eclades with at leas t50% hootstr aspupport,
Monophyly of the indica group is clearly demonstrate dT.he atalanta group is also tikel yto
be monophylctic. Although monophyly of Vanessa senstt stricto is i]ot shown in this analy-
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society oofJfap anJapan
Side-EffeMocdtel forNon-FunctionaTlraits 91
duced V. indica individua liss reminiscent ef tha tof normal I,lrnes sspaecies based on the
quantitati vvaelue of the relative area of orange (RAO) on the dorsa lforewing s(Ota l&ci
Yamamoto,2004h)CFig.IB),
In this normal species series, I noticed that the color of the subapical band embedded in the
black apical area is either orange (V ,samani (Hagen ,1895), V. dilect Haanafusa ,1992, and
V, tameamea Eschscholz, 1821 [althou jgt his sex-dependent in V. tameamea]) or white (V,
dcieani iGodart ,1824, V. betan a(Fruhsto r1f89e8r),, and V. atalanta (Linnaeu s17,58)) ex-
cept in V, indic awhose subapical band has both white and orange scales (Fi gI.B). The
color of the subapical band thus may be a usefu1 trai tto digtingui sthhe evolutionary tenden-
cy of expansion or shrinkage of the orange area in V. dilect aand V. buana, whose orange
area is not appreciably differen tfrem that of V, i.ndic a,Furthermere, the subapical band of
the V. indic aindividu awlith the expanded orange area induced by experimental treatments
is orange, although the band is cempromised in shape. On the other hand, the subapical
band of the V, indic aindividu awlith the colltracted orange area induced by experimental
treatments seems to be whiter than that of the normal individu a(lOtak iun,published data).
The subapical color of the modified individuals ,in addition to tbe relative area of orange,
thus faithfu11 ryecapitulates the normal series of I,keness sapecies. Hence, there is apossibil-
ity that lxtines ssapecies evolved by taking advantage of phenotypi cplastici otfy their com-
mon ancestral species that has been retained in V. indi. caand that can be revealed by physi-
sis, i tis suggested by Wahlberg et al. (2005 a)nd Fiel d(1971 ).(B )Coior patter mosf the
modified V. indica and the normal VZiness aspecies. The top pink panel shows the normal
V. indica (middl et)h,e modified V. indica induced by cold shock or its phenotypically
equivalent chemicals such as tungstate (righ tand), the ]nodified V. indica induced by heat
shock or it sphenotypicall ycquivalent chemicals such as thapsigargin (lef tT)he. heat-shock
and thapsigargin-iaject ieoxpneriments were published in Otaki (2008) .The subapi¢al hand
embedded in the black area at the apex in V. indica (purpl aerrow) is white with a small or-
ange area. The cQlor of this subapical band in a given $pecies correlates with the relative
area of orange (se ebelow), This modified color-pattern series is reminiscent of the norrnal
species series shown in the middle and bottom panels. The middle yello wpanel shows four
species that are molecular phylogenetica clllasysified as the indica group excluding V. indi-
ca itsel (fOtak eit aL,2006a). Compared to V. indica ,V. samani showsalarger orange area
and smaller black area together with an orange subapical band (oran garerow), whereas V.
dqieani sihows a smaller orange area and 1arge rb]ack area together with a white subapical
band (bla cakrrows). Two species, V. dilec taand V. buana do not show appreciably largcr
or smaller orange areas, compared to V. indica ,but they have either orange or white subapi-
cal bands (uran gaerrow and black arrow, respectjve!y). The bottorn blue panel shows t",o
species that are molecular phylogenetica lcllasysified as the atalanta group (Otak eit al.,
2006a) .Here again, V. tameamea has a relatively 1arg eorange area and small black area to-
g¢ther with the orange apical band (oran gaerrow), whereas V. ataZanta has a relatively
small orange area and larg eblack area together with the white apical band (blac karrow).
(C) Schematie diagrani of the possibl bei-directio cnoallor-pattern eyolution of Vtinessa
species. 1[1i cmolecular phylogenetic analysis indicat etdhut they are separated int otwo
groups :the atalanta group and the indica group (Otak eit al., 2006a). The color-pattern
analysis indicate dthat they can be classified into three types: the orange type, the intermedi-
ate type, and the black type (Otak eit at., 2006a) .From the hypothetic parlototyp ewh,ich
was probabl ysimilar to V, indica in tcnns of the wing color-pattern, at least four species, V,
tameamea, V. atalanta, V. samani and V. dq'eanii ,independently and allopatrically in-
creased or decreased the orange area on the forewing s.Other three specics, V. indica ,V.
dilecta ,and V. buana, basicall yretained the ancestral color-patterns.
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan
92 JojiM. OTAKI
ological experiments (Otak &i Yamamoto, 2004b) .It is worth noting that the possibl eevo-
Iutiona riymportanc eof phenotypi cplasticit iyn butterf icoylor-patterns has also been point-
ed out in a study of seasonal polyphenism (Shapi r1o9,76).
Thus fat rth,e site of action of the cold-shock agonists or antagonists has not been conclu-
sively determined. Circumstantia levidence suggests that beth neurosecretory cells and
scale cells may be afiiected by cold shock, and the scale cells are likel yto be affected by the
cold-shock agenist or antagonist iajectio n(sOtak i1,998; Otaki, unpublished data) ,but a
dilfere ngtroup proposed neurosecretory cells as an exclusive target (Serf a&s Carroll,
2005). More studies are necessary to resolve this issue,
Species relationships and the color-pattern yariation
Molecular phylogeneti rcesults (Wahlbe retg al., 2005; Otaki et at., 2006a) together with
synthetic morphological analyses (Fiel 1d9,71) help us to decipher the possibl eevolution-
ary histor yof the classical genus l,lrnes sseansu stricto. In Otaki et al. (2006a )se,ven
species of Itlanes wserae analyzed excluding V, vulcania Godart ,1819, which was previously
recognized as a subspecies of V. indica (Fiel 1d9,71; Leestmans ,1978) ,and V. abyssinica
(Feld e&r Felder ,1867) ,which was previousl yrecognized as Antanartia abyssinica
(Wahlbe ertg at,, 2005) and whose taxonomic position in 1ilanes snaeeds to be so]idified by
furthe rintegrati svteudies. This paper hereafte arlso focuse son these seven species of the
genus S,2xness saens" stricto.
There are phylogenetical tlwyo groups in Iikeness a(:l )the atalanta group ,composed of V.
ataianta and V. tameamea, and (2 )the indica group ,composed of the rest of the ;,kenessa
species (Otak eit al., 2006a) .The phylogeneti tcree of the indica group is shown as V. indica
(V ,samani (V .dojeani (iI Ldilect +a V. b"ana)) )(Fi gI.A). The monophyly of the indica
group is clearly demonstrated .The atalanta group is also likel yto be monophyletic, al-
though not strongly supported. The molecular phylogeneti canalyses by ourselves and
other rescarchers (Wahlber egt at., 2005; Otaki et al., 2006a) are basical lcyompatible with
the synthetic morphological analyses of Field (1971),
In contrast, the color-pattern phenotype of the genus Iikenes scaan be classified into three
types 1argel byased on the relative area of orange (RAO) on the dorsa lfbrewings: (1 )the or-
ange type, whose orange area is relatively large ,i .e, V. tameamea and V. samani, (2 )the
black type whose orange area is relatively small, i .e, V, del'ean ianid V. atalanta, and (3)
the intermedia tteype, i .e. V. indica ,V. buana, and V, dilect a(Otak eit al., 2006a), This
area of orange in lrknessa is slightly but significantly differe nftrom species to species
(Ota k&i Yamamoto, 2004a). [[ bbe sure, other ce]or-pattern trait sare also atfected by the
CSH and may be considered fbr a similar grouping purpose ,but the orange area en the dor-
sal wings is the most conspicuous at leas tto hurnan eyes and probably also to the eyes of
i/Ziness bautterfli ebsased on the molecular and behaviora dlata (Zaccar edt ial.., 2006) .It is
to be noted also tha tthere is no conspicuous sexual dimorphism in the Iikness aspecies.
The functiona slignificance of the orange area has not been strictly examined in leinessa,
However, it is known that Y. indic aprimarily uses color visual cues rather than olfactory
cues for flower yisitation (Omur a& Honda, 2005), indicatin gthe function ailrnportan cofe
the visual system in this butterf lbyehavior ,Interesting lVy, ,atalanta cannot discriminate
colors with long wavelengths, between yellow (59 0nm) and red (62 0nm), althoug hit can
do well between blue (44 0nm) and yellow C59 0nm) (Zacca retd ait., 2006) ,indicati ntghat
V, atalanta and probabl yalso other species of l,kenes sraecognize the color contrast (i e., or-
ange and black )but not the color itse] fin the long wavelengths of light ,In a different
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan
Side-Effect Model for Non-Functjona Tlraits 93
nymphalid butterf lthyat has a similar broad orange stripe patter rwi)th black background on
the wings, }bma sabina, an experimental study showed that the orange stripe with black
background is importan tin the visual recognition (Warzec g&a Ege]haaf ,1995). We can
thus presume that the orange area on the dorsal forewings, when combined with a black
background ,preduce shigh contrast, and this visual signal functions as a mating signal in
l2iness ad,espit ethe fac tthat i,lanes bsuatterfi icaensnot recognize the orange color itself.
The combination of the phylogeneti cand color-pattern analyses revealed an interesting
trend (Otak eit al., 2006a) ,The indic agroup contains one species of the orange type (i e,.
V, samani), one species of the black type (i e.. V, dcieanii )an,d three species of the interme-
diat etype (i e., V, indica ,V. dilect aa,nd V, buana) .Likewise ,the atalanta group contains
two species with quit edissimila rcolor-patterns: one species of the orange type (i e.. V.
tameamea) and one species of the black type (i e.. V. atalanta). This immediately indicates
that the color-patterns based on the orange area do not simply reflect the molecular phylo-
geneti crelations and the classification of Field (1971 b)ased on genita land other morpho-
logical
characters,
Such disagreement between the phylogeneti sctatus and the color pattern iss not surprising
at all in butterfli eBsu.tterfl ycolor-patterns can be drastical dliyffere nbettween closely-re-
late dspecies and even within a single species as exemplified by lhpili odardanus, Ptipilio
memnon, and Hiypolimnas hottna (Nljhou t19,91). Also color patterns can be very similar
even between non-related species as seen in many pair sof mimics and their models, For
these reasons, wing color-patterns are not considered as very usefui traits for clarifying pby-
logeneti rcelations between butterf ispyecies, This widely accepted fac tstraightforwardly
indicate sthat the wing color-patterns of butterfii enost, excluding 1,ixnes shaa,ve high de-
grees of freedom in developmen tand evolution despit ethe existence of the nymphalid
groundplan, which is the genera lframework for butterf iwiy'ng color-patterns (Schwanwisch,
1924; SUffer t1,927; Nljhout, 1991) .The combined result of the phytogeny and the color-
patter nanalysis readily indicat etshat also in l/lrnes esvean within a group of closely related
relatives, the color-pattern can drastical vlayry. However, despit ethe color-pattern differ-
ences arnong species, the variation is always on the lin eof the simple color-pattern series:
the expansion or shrinkage of the orange area.
Bi-direction acollor-pattern eyolution
For the sake of discussion ,here I would lik eto introduc ea presumptio nthat the ancestral
species of vauzess haad a color patter nsimilar to V, indic a(Otak eit at., 2006a) .This pre-
sumption, which does not contradict the molecular phylogenetic data (Wahlber get al.,
2005; Otaki et al., 2006a) ,is mainly based on the fac tthat V. indic aoccupies the middle
posttio nin the linea rseries of the quantitati cvoelor-pattern differenc ebsased on the RAO
values (Otak iet, al., 2006a) .In addition, V. indica is widely distribut iend Asia, indicating
it sgeneti cversatility to adapt itse ltfo differe nentvironmental conditions, compared to other
I,kenessspaecies,
I believ ethat iilrnes sspaeciation was a typica lallopatric process, as proposed in Mayr
(194 21,963) ,because many of the presen tIrti.nes sspaecies show restricted patchy distribu-
tions in isolate idsland s(Fi gI.C). Exceptions are V. indica and V atalanta, both of which
are distribut ewiddely in the Eurasia n(V ,indic aand V. atalanta) and North American (V.
atatanta) Continents and are sister to islan dspecies. Thus, it is highl ylikel ythat the ances-
tral species was found on a continent and was closer to V. indica and V. atatanta. In the
course ef speciation, this hypothetic aalncestral species was geographical liysolate din a
small island ,and after many generatio nwsith no interbreedin gwith other populations of the
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan
94 Joji]vlO.TAKi
ancestral species, geneti cdilferenc ewesre accumulated in the isolate pdopulatio nw,hich
eventually changed some trait sthat were related with sexual reproduction includin gthe
wing co]or-pattern. Four species, V, tameamea, V, samani, V. atalanta, and V. dojeanii,
were differentiat ewdith clearly appreciable expansion or shrinkage of the orange area (Fig.
1C), and the other three species, V, indica ,V, ditect aand V. huana, simply retained'a simi-
1ar extent of the orange area and most of the color-pattem elements.
This "bi-directional" type of color-pattern evolution (i e.. expansion or shrinkage of the or-
ange area) is clearly obseryed only within the genus Vinness saensu strictv, It is not clearly
observed in Cynth iora Bassaris .However, some nymphalid genera such as N.vmphatis liy-
ing in high altitude areas are likel yto show similar evolutionary tendencjes (Otak iun,pub-
Iished data) ,Interesting tlhyi, stenclency is seen irrespecti ovfe the two phylogeneti gcroups
(i e., the indica and atalanta groups )and also irrespecti ovfe geographic allocatio nosn the
earth, Other than the change of the Qrange area, no drasti pchenotypic evolution had hap-
pened jn vainess sauch as the innovatio onf eyespots on the dorsa lwings as seen in lanonia
or the unequal expansion of eyespots on the ventral hindwings as seen in C.ynthia,
It is rme that Ciynthi aand Bassari sare also found in high altitude areas in tropical latitudes
as well as in temperate areas, Why the bi-directiona liis tobyseryed only in lxinnes bsuat not
in C>'nth iaand Bassaris is unclear at this point ,But it would sufice to mention here that
the directio nof the phenotypi ccolor-pattern divergence of species in lhnessa appears to be
pre-detennin eads an evolutionary constraint at the genus level ,In other words, this con-
straint is what characterize irlanes asnda distingui siht from other genera.
Based on the discussi oanbove, an explanation that this bi-directio nteanldency of the color-
pattem changes is merely a product of random geneti cdrif tor stochastic events tha thap-
pened in each specjes independentl wyith no pre-determine cdonstraint cannot be justified.
This evolutionary constraint being unique to iiltness maay have played an instmctiv reole in
the color-pattern evolution in Llaness aE,ach differentiati npgopulation might have had the
freedom to set the leve lof the orange in accordance with the environmental conditions (see
be]ow).
Although the possibili tthyat the bi-directio ncoallor-pattern evolution might have been di-
rectly shaped by natural selection cannot be ruled out completely, it is at leas tequally rea-
sonable to think that the smal1 increas eor decreas eof the orange area vv"as eco]ogically too
trivial to be selected fbr in the early stages of the specific differentia toifo in,2xness aI,t is to
be noted that whether or not the present lthness aspecies can discrimina ttehe interspecific
RAO diiference sdoes not either deny or support this idea,
Tb be sure, the orange area itsel fis likel yto be behaviorally important as discusse dabove,
but it ssmall bi-directio nmoadlifications (i e.. increas eor decreas eof the orange area)
would not be able to confer any ecelogical advantage on the ancestral species of l,kenessa,
even if the optimal width of the orange area may be behavioral liymportan tin some of the
presen tspecies of Vlrness a.It is also important to notice that most, if not a]1 ,I,lvnessa
species certainly evolved allopatrically, judgi nfgrom their patchy distributi poanttern in iso-
lated islands (se aebove; Fig .IC). In other words, the ancestral color-pattern would be just
good enough for mating recognition in the isolate ednvironment where no other closely re-
Iated species existed, Furthermore, since there is no clear sexual dimorphism in color pat-
terns in Lirness a(an exception is the subapical band color in V, tameamea), it can be con-
cluded that sexual selection did not play a central role in the course of the specific differen-
tiation of this genus, although an assortative mating proces smay be necessary to spread a
particula rgenotype that supports one of the plasti pchenotype sin the isolate pdopulation
(seebelow;Fig.2A).
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan
Side-EffecMtodel forNon-FunctionaTlraits 95
Physiologic allink with the cold-shock resistance
It can be argued as above that the origin of the interspeci fRiAcO differenc eansd hence the
bi-directiona clolor-pattem evolution cannot be reasonably found in it secological and sexu-
al functionalit yT.his is not surprising in that not a]] butterf lcoylor-patterns have clearly
identifia bfluenctio nat least to biologist s.Rather, many butterf icoylor-pattems are simply
extravagant with many non-functional pattern elements (se eIntroduction) a,nd I believ ethat
lrkenes issa not an exception. Then, how did the bi-direction aclolor-pattern evolution origi-
nate in ltlrnes srlab ? ・put it difierent lwhyat, is the real facto rbehind the evolutionary con-
straint of the genus 1/2znessa?
[Ib resolve this problem ,the color-pattern evolution can be considered as a consequence of
natural selection fOr a seemingly unrelated ecological trait .That is ,in association with the
eyolutionary constraint or phenotypi cplastici dtiyscusse din the previous section, a novel
color-pattern might have occurred as a by-produc tof the proces sof physiologic asellection
for new environmental conditions in which the cold-shock or heat-shoc kevents frequent]y
occur. To put it differentl yp,hysiologic aorl geneti cchanges for a new environment via nat-
ural selection might have caused the color-pattern medificatiens and evolution as a side
effect, because of the opportunistic physiological link between the cold-shock resistance
and the color-pattern modifications, This is consistent with the fac tthat the genus iilanessa
is composed of species that are adapted to environmental temperature fiuctuation sin tem-
peratearea,
In this "physiological side-etfect medel" (Fi g2.A), an ancestral populatio nis firs etxposed
to a new environment with high]y fluctuatin gtemperatures. For the eMcient protecti oonf
differentiat piunpgal cells against the environmental cold shock, the putativ eCSH is pro-
duced into the hemolymph. The ability to produce the hormone eMciently varies among in-
dividua ldsue to differe ngtenetic composition. The environment primaril syelects individu-
als with high cold-shock resistance based on the eMcient production of the CSH,
However, this hormone, if secreted during the formation of the wing color-pattem, has an
opportunistic ability to modify the color-pattern determinati opnroces sof scale cells, lead-
ing to the wing-wide color-pattern modifications of the individua lT,hat is ,as a sicle effect
of this hormone, the wing color-pattern is modified, and as a result, the number of individu-
als with "aberrant" celor-patterns increase isn the isolate pdopulatio n,In other words, the
morphological diversi twiythin that population is promoted ,as seen in a natural population
of Zi.7eeri maaha (Koll a1r84,4) exposed to a new cold environment (Kud o& Ichida ,2002;
Otaki & Kudo, unpublished data).
If the highl ymodified color-patterns are not attractive to mates (show nas (1 )in Fig, 2A),
the mating proces snegatively affects the selection step for the cold-shock resistance by not
favoring the individua lwisth high hormonal activity. As a compromise, individua lwsith
"appropriate" hormonal activity may survive bette rthan those with high hormonal activity.
The survjvors rnay show relatively small but significant color-pattern modifications. This
way, both the physiologica lphenotype (i e.. the co]d-shock resistance) and it sopportunistic
phenotype (i e., the modified color-patterns) as a by-product are geneticall yassimilated in
thepopulation.
Not al 1color-pattern modifications will have a negative effect on mating, because not all
color-patterns or their elements can functio nas mating signals and in many butterfi ioenlsy
the rough pattern sare thought to be function a(lse eIntroductio n)I.t would often be the
case that the color-pattem rnodifications are not even recognized by mates, because of the
NII-Electronic Library Service
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society oofJfap anJapan
96 JojiM,OTAKI
A
Migration to the high alntud eaTetts :GeOgrseek{Eo8nl
+
Exposuretocotdshack
Rcvelation hormone
ofphenotypic plastici toyf cold-sheck
NN<g:Le
eftect
Revelatio nofphenotypic plastici tofy color pattems
(Color-patterri
modifications as a side effect)
Selecti ofnbr cold-shock resistance IU (( 1".2O.1:i,a,9a:t.iV.effL",htibitiOn
+
(3 )adaptivei acceleration
C ocmolpdr-oshmoicksroedsis tganecneeti ac ssAiNmDilat ieons ,of.,.,. Ials"iepeaee.,,.,i,,l.tfu,,,,,,,1es1',\.
? i: (opportunisticphenotype)
-lii[ve
Gy
Assorta mating F,".". ec..';`:,i.,,
A
stable new species
B
coid-schok <e-,N.aturaSleleCtiOn
resistance
with the'i C)SH)`i>p,;:'X:gl"'Sl:ltll"'"ag "iii" j' 3/
/ /, s.:n.,::.ge
sideeffet/.l{IIill,,,,l,, c
.i,,,ri,l/,,i.,,ie/lnl,
,,,.sid
//,,..g,///,ee,l'l・i'I.l#.igg'..Ff..{.gr.?leietrge,i..//・,l.1il,ite.l.,I.,,/・C....
Fig, 2. Physiologica lside-effect model for the color-pattern evolution of Vdnessa butterfiies,
(A) Evolutio onf Vbnessa color-patterns. In this model, the ancestral populati otnhat mi-
grated to high altitude regions is geogrttphical liysolat eadnd exposed to natural cold-
shock cvents. In response to the cold shock, the cold-sheek hormone (CSH) is produced
into the hemolymph, Phenotypi pclastici toyf hormone productio mnay be revealed by
thi sproces s.In addition, the produced horrnone modifies the wing color-pattern as a
side effect, although it sprimary function is to protec tdifferentiati ncgells from cold
shock. Here, phenotypi pclastic ioftthye wing color-patterns is revealed, resulting in the
phenotypical dliyvers epopu]atio nA.t this point ,three cases may be considered. (1)
The produce dmodification may be non-adaptive. That is ,the modified color-pattem is
not attractive to mates, which inhibi tthse positi vseelection for cold-shock resistance. If
highly adyerse, the modification may entirely stop the selection step fOr cold-shock re-
sistancc, and such a population may die out. Il 'not so adverse, thi sstep may inhib itthe
selection only slightly, and the modification may accompany the evolution of cold-shock
resistance. (2 )The produced modification may be neutral with no adverse effects and no
adaptive valucs. in this case, the modified celor-pattern simply accompanies the selec-
tion for cold-shock resistance, (3 )The produce dmodifications may be adaptive in rare
cases. In that case, selection for the cold-shock resistance will simply be accelerated by
the adaptive selection for the color pattern .In any case, if the populution does not die
out, the result is the genetic assirnilation ef thc cold-shock resisLance and the modified
NII-Electronic Library Service