Phylogenetic Relationships and Taxonomic Status Of the Paleoendemic Fagaceae Of Western North America: Recognition Of A New Genus, Notholithocarpus PDF
Preview Phylogenetic Relationships and Taxonomic Status Of the Paleoendemic Fagaceae Of Western North America: Recognition Of A New Genus, Notholithocarpus
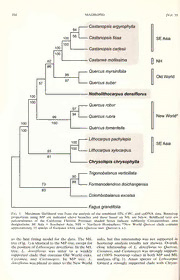
Madrono, Vol. 55, No. 3, pp. 181^190, 2008 PHYLOGENETIC RELATIONSHIPS AND TAXONOMIC STATUS OF THE PALEOENDEMIC FAGACEAE OF WESTERN NORTH AMERICA: RECOGNITION OF A NEW GENUS, NOTHOLITHOCARPUS Paul Manos S. Department of Biology, Duke University, Box 90338, Durham, NC 27708 [email protected] Charles H. Cannon Department ofBiological Sciences, Texas Tech University, Box 43131, Lubbock, TX 79409; Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan 666303, China [email protected] Sang-Hun Oh L. H. Bailey Hortorium, 412 Mann Library, Cornell University, Ithaca, NY 14853 [email protected] Abstract We investigated the phylogenetic relationships and taxonomic status ofthe castaneoid component {Lithocarpus and Chrysolepis) of the family Fagaceae that is endemic to the California Floristic DNA Province (CA-FP). Over 7800 basepairs ofnuclear and chloroplast were analyzed in 17 taxa representing the breadth ofphylogenetic diversity in the family. The genus Lithocarpus^ as currently defined, isclearlypolyphyleticduetotheinclusion ofL. densiJJorus. Here, wedesignatethistaxonasa new genus, Notholithocarpus, which can be recognized morphologically by its relatively small, subprolate pollen. Notholithocarpus is more closely related to Quercus, Castcinea, and Castcmopsis; Chrysolepis was resolved as the sister group to Lithocarpus sensu stricto. These results indicate that Notholithocarpus does not possess true 'flower cupules,' which define Lithocarpus sensu stricto, but liketheoaks, thesinglefiowerpercupuleisderivedthroughtheabortionoflateralflowerswithineach cupule. Further study is required to confirm this characteristic. A formal taxonomic treatment is presented with new combinations. Key Words: California Floristic Province, Chrysolepis, Fagaceae, Lithocarpus, Notholithocarpus, phylogeny, sudden oak death, tanbark oak. The Cahfornia Floristic Province (CA-FP) of florus var. densiflorus (Hook. & Arn.) Rehder, a western North America is rich in paleoendemic tree that reaches 45 m, often occurs as a co- plant species that highlight the botanical legacy dominant in the redwood and mixed evergreen of the region and stimulate discussion on the forests ofthe north coast ranges; its shrub form, affinities ofmany ofthese putatively isolated taxa L. densiflorus var. echinoides (R. Br. ter) Abrams (Stebbins and Major 1965; Raven and Axelrod is more common in open conifer forests and dry 1978). Among these taxa are representatives of slopes of the northern interior CA-FP at higher the largely tropical East Asian castaneoid sub- elevations. The giant chinquapin, Chrysolepis family of the Fagaceae: Chrysolepis Hjelmq. and chrysophylla (Douglas ex Hook.) Hjelmq., a tree Lithocarpus Blume. Both are morphologically potentially reaching 50 m, also occurs mostly in similar to other broadleafevergreen Fagaceae of redwood and mixed evergreen forest. The bush eastern Asia, but their phylogenetic position chinquapin, Chrysolepis sempervirens (Kell.) within Fagaceae is either unresolved or has been Hjelmq., is more common at higher elevations put into question with recently available data. In as a low-growing shrub on rocky slopes under this study, we address the relationships of the coniferous forests ofthe Sierra Nevada and in the castaneoids of the CA-FP and discuss the isolated interior ranges ofsouthern California. implications of our findings in the context of Taxonomic delimitation of the castaneoid taxonomy, cupule evolution, and biogeography. genera is based largely on characters of the The two castaneoid taxa in question are well pistillate flower and cupule. The most important represented in several CA-FP communities (Bar- morphological characteristic for defining Chryso- bour and Minnich 2000), and both express lepis is a cupule with internal valves separating sufficient differentiation in habit and leafmorphol- the fruits (Hjelmquist 1948). This condition is ogyfortherecognition oftaxaatthesubspecific-to autapomorphic within Fagaceae, and the valves species-level. The tanbark oak, Lithocarpus densi- have been interpreted as vestiges ofthe branches MADRONO 182 [Vol. 55 ofhigher-order inflorescences (Nixon and Crepet Whilethecombination ofreproductivefeatures 1989). Both chinquapin species originally were observedinL. densijlorusfitscomfortablyintothe treated in the chestnut genus Castcmea Miller, range ofvariation observed in Asian Lithocarpus, and later transferred to the strictly Asian genus non-floral studieshave suggested otherwise. Most Castanopsis(D. Don) Spach, consistent with their Asian Lithocarpus possess 2 to 4-rayed thick- evergreen habit, leafvenation, and spiny cupule. walled trichomes, while L. densijlorus has distinct Hjelmquist's (1948) segregation of the chinqua- multiradiate thin-walled trichomes (Jones 1986; pins into Cluysolepis was supported by Jones' Cannon and Manos 2000). In addition, previous (1986) foliarinvestigation ofthe family. He noted molecular phylogenetic studies showed that L. that compared to Castanopsis, Chrysolepis has densijlorus does not form a clade with species of smaller leaves and unique thick-walled peltate Asian Lithocarpus, one of the most strongly trichomes, likely adaptations to xeric conditions. supported groups in all of Fagaceae (Manos et Recent phylogenetic studies also have supported al. 2001); the placement oftanbark oak remained this distinction, resolving Castanea and Casta- largely unresolved in that analysis. nopsis as sister genera to the exclusion of In this study, we clarify these lingering !' Chrvsolepis (e.g., Manos et al. 2001; Oh and questions about the phylogenetic relationships Manos 2008). among the castaneoid taxa of the Fagaceae, The taxonomic history of Lithocarpus densi- particularly in relation to the endemic CA-FP florus is relatively straightforward. The species taxa. Our analysis involves three main goals: 1) | was recognized first within Qiiercus, with the phylogenetic reconstruction of a broad sampling following commentary: ''This remarkable plant of Fagaceae, representing all major lineages, DNA has very much the appearance ofa Castanea, the using multiple sequence data sets; 2) fruit in the only specimen we possess being analysis of pollen morphology of L. densijlorus situated at the base ofa male somewhat fascicled to further elucidate its relationship to Asian catkin of the former year, while the numerous Lithocarpus; and 3) taxonomic revision of L. male catkins of the present year present no densijlorus based upon these results. appearancewhateveroffemale flowers." (Hooker and Arnott 1840, p. 391). The acorn-like fruit of Materials and Methods L. densiflorus develops biennially from a single pistillate flower surrounded by a valveless cupule. Molecular phylogenetics Miquel (1857) first introduced Qiiercus subg. Pasania in a treatment of south Asian species of Seventeen taxa representing all ofthe currently Fagaceae and Orsted (1866) later raised the recognized genera and major lineages of Faga- subgenus Pasania to the generic rank, wherein ceae were chosen for study based on previous Pasania c/ensiflora (Hook. & Arn.) Oerst. was phylogenetic analyses (Manos and Stanford 2001; treated together with a mixture of largely Asian Manos et al. 2001; Oh and Manos 2008). species under subg. Eupasania. Although no type Previous analyses addressed intra- and interspe- has been associated with the name Pasania, the cific samplingofthecastaneoids oftheCA-FP, as name is often misapplied to Asian species of well as Lithocarpus and Castanopsis. The current Lithocarpus (Prantl 1888). Rehder (1917) treated sample is designed to focus on the broader Pasaniadensiflorawithin Lithocarpus, and Camus relationships within Fagaceae using more data. (1936-1954) placed L. densiflorus within Litho- The names, authorities, sources, geographic carpus subg. Pasania, in the monotypic sect. origin, and GenBank accession numbers are Androgyne. summarized in Appendix I. The genus Fagus is Historically, there has been little doubt about consistently recognized as sister to all remaining the relationship of L. densijlorus to the approx- Fagaceae; it is used here as the outgroup (Manos imately 250 species of Asian Lithocarpus (stone and Steele 1997; Li et al. 2004). oaks). The genus concept is supported by the We included two nuclear loci (ITS region and flower-cupule described by Forman (1966a, b), CRABS CLAW or CRC) and three chloroplast wherein each pistillate flower of a dichasium is regions {trnK-matKltrnK, atpB-rbcL and ndhF). surrounded by a distinct valveless cupule. As a The CRC data were taken from Oh and Manos whole, the genus Lithocarpus shows abundant (2008) and the nuclear ITS and chloroplast trnKJ variation in inflorescence structure, sexual condi- matK and atpB-rbcL data were obtained from tion of the flower spikes, numbers of staminate Manos and Stanford (2001) and Manos et al. and pistillateflowersineachdichasium, fruittype, (2001). Some sequences of the trnKlmatK region and cupule type and ornamentation (Kaul 1987; and most of atpB-rbcL sequences were newly Cannon and Manos 2001). In L. densijlorus, the generated here, using the methods described in inflorescences may be androgynous with pistillate Manos and Steele (1997) and Manos and flowers at the base, orentirely staminate. There is Stanford (2001). The ndhF region was newly a single pistillate flower per dichasium, and the sequenced in this study, using the PCR primers cupules bear strongly reflexed scales. ndhF972 (5'- ATG TCT CAA TTG GGT TAT 2008] MANOS ET AL.: PHYLOGENY AND TAXONOMY OF PALEOENDEMIC FAGACEAE 183 Table 1. Specimens Usedinthe Pollen Study. ssiwmapplpeingseiqnuePnAcUeP*a.ddTithieonbesatnfdittTinBgRevoblurtainocnh- ML NotJwlithocarpiis deusifloi-us, California, Butte Co., ary model in the analysis was determined by border ofponderosa pine, Douglas fir forest, 5 mi S the hierarchical likelihood ratio test using Mod- ofSterling City, 12 Jul 1968, Terrells.n. (US); eltest 3.06 (Posada and Crandall 1998). Humboldt Co., Seely Ranch, ca. 2 mi E ofWillow ML bootstrap analyses were conducted with (sClrDoeUpeeKkEo)ofn;ritMdhgeeenTSdroEicnoiiftnyRoeRCidov.eM,rt,.a,l3o8JnuJglulR1e917d917,0M,tS.Ctloranoueasd3e,n03N740- G50A0RLpIseudvoerrseipolnica0t.e9s51 by(Zwuisciknlg 2t0h0e6).prAogrsaixm- 91 (NY); Oregon, Curry Co., Babyfoot Lake on the substitution parameter model was employed to upper drainage ofthe Chetco River, 22 Sep 1964, calculate a likelihood value. Empirical base Chambers2277 (US) frequencies were used, and other parameters, including the shape parameter of gamma distri- Chrysolepis chrysophylla. California, Shasta Co., E-face ofCrater Peak, 8200 ft, volcanic, subalpine, 16 Aug bution and proportion of invariable sites, were 1964, /. Olmsted259 (DUKE). estimated from the data. Default values were LitRheohcdaerrp;usCdheianlah,atSuisch(uHaono,k.Yfon&g-JTihao:mMsaonnoesx1M2i8q8.) useWdefortegsetneedticthaelgmoorintohpmhyanldy otohferListethtoicnagrs.pus, (DUKE) including L. densijlorus, using the Shimodaira- Hasegawa test (Shimodaira and Hasegawa 1999; CasYtuannnopasni,s Minednigcalu(mR:oxMb.aneoxsLiannddleZyh)oAu.1D42C6:(CDhiUnKaE,) SH teMstP), as implemented in PAUP*. For the SH test, trees generated enforcing the topological MP constraint were evaluated against the original ATG ATG; Olmstead and Sweere 1994) and tree based on 10,000 bootstrap pseudoreplicates FndhF2110R (5'- CCT CCT ATA TAT TTG using the re-estimated log likelihood (RELL) ATA CCC TCT CC) with Phusion High-Fidelity method. DNA polymerase (New England Biolabs, Ips- wich, Massachusetts, USA) under the following Palynology conditions in 25 \xL reactions: initial denatur- ation at 98 C for 1 min 30 s, 40 cycles of98 C for The pollen of four specimens of Lithocarpus 10 s, 60 C for 30 s, and 72=C for 1 min, followed densiflorus collected in California and Oregon by final extension at 72 C for 7 min. PGR was examined, in addition to representative products were purified with the QlAquick PGR species of other castaneoid genera (Table 1). Purification kit (Qiagen, Valencia, California, Pollen grains were acetolyzed using the method USA). All sequences were deteimined at the in Faegri and Iversen (1950). Specimens were DNA sequencing facility at the Duke Institute for mounted on stubs, coated with gold palladium DGeNnAomAenaSlcyizeenrce(sApapnldiedPoBliiocsyysttheatmsu,sFeosstaer37C3i0txy1, woibtsheraveHduamnmdeprho6t.2ogsrpuatptheerdcuosatienrg(aAnPhaitleicphs)X,La3n0d California, USA). Sequences were edited in ESEM TMP (FEI Company) in the Department Sequencher version 4.5 (Gene Codes Corpora- of Biology SEM facility of Duke University. We tion, Ann Arbor, Michigan). also measured polar and equatorial diameter and Sequences were manually aligned using Mac- calculated the P/E ratio for 30 pollen grains ofL. Clade version 4.06 (Maddison and Maddison densiflorus with light microscopy (LM) using the 2000). Phylogenetic analysis of the combined following collections: Terrell s.n. (US), Clausen data was conducted with maximum parsimony 70-91 (NY), and Chambers2277(US). The pollen (MP) and maximum likelihood (ML) methods. was mounted and stained with lactophenol- Eighteen sites in intron four of CRC were cotton blue and viewed under a Leitz microscope excluded in the analyses because of alignment at lOOOX magnification. ambiguity. All other characters were treated as MP unordered and equally weighted in the Results analyses. Gaps resulting from multiple alignment of indels were treated as missing data. In both The final alignment ofcombined data included MP ML and analyses, heuristic searches in 7866 characters of which 1022 were parsimony- PAUP* (Swofford 2002) were used to find the informative. The alignment was deposited in best scored tree with 100 replicates of random TreeBASE (URL: http://www.treebase.org/). taxon addition and tree bisection-reconnection The MP analysis produced a single tree with a (TBR) branch swapping, saving all of the best length of 1946 steps, consistency index of 0.573 trees at each step (MulTrees). Branches with a (excluding uninformative characters), and reten- minimum length of zero were collapsed using tion index of 0.643. ModelTest selected the "amb-" option during the searches in the MP general time-reversal model (GTR; Swofford et analysis (Nixon and Carpenter 1996). Bootstrap al. 1996) with six rate parameters, the gamma analysis (Felsenstein 1985) using the MPcriterion distribution parameter (F), a = 0.793, and with 500 pseudoreplicates was conducted with proportion of invariable sites (Pinvar) = 0.4386 MADRONO 184 [Vol. 55 Castanopsis argyrophylla 54 56 100 Castanopsis fissa SEAsia 100 100 Castanopsis carlesii 100 62 Castanea mollissima NH D 86 Quercus myrsinifolia 99 Old World 97 57 Quercus suber Notholithocarpus densiflorus 100 100 Quercus robur 97 99 94 Quercus rubra New World* 100 100 Quercus tomentella 100 Lithocarpuspachylepis 100 SEAsia 100 93 Lithocarpus xylocarpus 81 Chrysolepis chrysophylla Trigonobalanus verticillata 90 88 73 Formanodendron doichangensis 83 Colombobalanus excelsa Fagus grandifolia Fig. 1. Maximum likelihood tree from the analysis of the combined ITS, CRC, and cpDNA data. Bootstrap proportions using MP are indicated above branches and those based on ML are below. Boldfaced taxa are paleoendemics of the California Floristic Province; shaded boxes indicate subfamily Castaneoideae; area designations: SE Asia = Southeast Asia, NH = Northern Hemisphere; *New World Quercus clade contains approximately 15 species ofEurasian white oaks {Quercus sect. Quercus s. s.). ML as the best fitting model for the data. The oaks, but this relationship was not supported in MP tree (Fig. 1) is identical to the tree, except for bootstrap analysis (results not shown). Overall ML the position ofLithocarpusdensiflorus. In the close relationship of L. densiflorus to Quercus tree, L. densiflorus was sister to a weakly Castanea, and Castanopsis was strongly support- MP ML i supported clade that contains Old World oaks, ed (100% bootstrap value) in both and MP i Castanea, and Castanopsis. In tree, L. analyses (Fig. 1). Asian species of Lithocarpus densiflorus was placed as sister to the New World formed a strongly supported clade with Chryso-l 2008] MANOS ET AL.: PHYLOGENY AND TAXONOMY OF PALEOENDEMIC FAGACEAE 185 lepis. Forcing the monophyly ofLithocarpus and (Crepet and Daghlian 1980; Wang and Chang L. densijlorus required 48 additions steps, and the 1989; Fig. 2). In contrast, the pollen of Notho- SH test indicated that the constrained topology is lithocarpus is distinctly smaller and rounder than significantly worse than the original tree (P < the grains of Lithocarpus s.s. (mean P/E ratio of 0.0001). 1.68), and appears to be among the smallest and The pollen of Lithocarpus densijlorus is tricol- most spheroidal grains (P/E ratio of 1.2) thus far porate and subprolate (Fig. 2A) with an irregu- observed among extant castaneoid species larly striate to smooth exine (Fig. 2B). On the (Fig. 2). In summary, we consider the distinct basis of examining 30 pollen grains under LM, subprolate shape of the pollen grains of Notho- the average measurement was 17.8 X 12.7 \xm lithocarpus combined with its unique multi- (polar = 17.2 |Lim, minimum and 18.8 |Lim max- radiate leaf trichomes not found in Lithocarpus imum; equatorial = 9.42 |am, minimum and s.s. (Jones 1986)anditsphylogeneticplacementto 14.1 |am maximum), and the average P/E ratio be strongevidence to recognize this paleoendemic was 1.4. The pollen was smaller under the SEM ofthe CA-FP as a separate genus. (12.5 X 10.4 |Lim), but the P/E ratio under both Our robust phylogenetic reconstruction of the conditions was similar (1.4 in light vs. 1.2 in Fagaceae provides strong evidence of the para- SEM). Apparently, the pollen grains increased phyly of subfamily Castaneoideae (Fig. 1) with LM slightly in size afterthe initial preparation for major implications for our understanding of because of fluid uptake. The pollen grains of cupuleevolution (Fig. 3). Support for Chrysolepis other representative castaneoids were prolate (Fig. as the sister genus to Lithocarpus s.s. combines 2C-H): Chrysolepis (P/E = 1.8), Asian Lithocar- two genera that each possess well-defined cupule/ pus (P/E = 1.6), and Castanopsis (P/E = 1.5). fruit apomorphies. One possible synapomorphy for this relationship is the flower cupule {sensu Discussion Forman 1966a), where each pistillate flower in a dichasium is surrounded by cupular tissue. The Phylogenetic analysis of over 7800 nucleotides placement of the new genus Notholithocarpus resolves the placement of the castaneoids of the within the Quercus and Castanea + Castanopsis CA-FP into two distinct lineages ofthe emerging clade suggests that its single-fruited cupule is phylogeny of Fagaceae (Fig. 1). Chrysolepis is derived from reduced dichasial cupules via loss of strongly supported as sister to Asian Lithocarpus; lateral flowers (Fig. 3). Developmental data from while Lithocarpus densijlorus, hereafter referred Quercus indicates two initiation points or pri- to as Notho/ithocarpus densijlorus (formal taxo- mordial cupule valves subtend the pistillate nomic treatment is provided below) is placed flower (MacDonald 1979), which represents a within the Quercus and Castanea + Castanopsis different ontogenetic process than the completely clade, several nodes away from Lithocarpus s. s. valveless cupule of Lithocarpus s.s. (Okamoto These results clearly indicate the polyphyly of 1989). Further studies are needed to confirm Lithocarpus s. 1. A similar topology with high these evolutionary patterns of cupule develop- bootstrap support resulted from analysis of ment inferred from our phylogenetic analysis of nuclear sequences (CRABS CLAW; ITS) with a DNA sequence data. larger sample oftaxa (Oh and Manos 2008) and a Delimitation of the castaneoid genera can be previous analysis of only the ITS region across difficult, as few traits are truly fixed and exclusive Fagaceae, including over 50 species of Lithocar- to a single genus. Forexample, while most species pus s.s. also provided strong support for the of Castanea and Castanopsis possess three monophyly Lithocarpus s.s., but no close rela- pistillate flowers per cupule, certain species in tionship to Notholithocarpus (Manos et al. 2001). these genera have a single pistillate flower per Pollen morphology and ultrastructure have cupule, a trait that normally defines Lithocarpus provided important systematic data to classify s.s. (Fig. 3). A combination of traits is often modern Fagaceae, aswellastoassesstheaffinities needed and Notholithocarpus is no exception. In DNA offossil taxa (Crepet and Daghlian 1980; Crepet addition to sequences and pollen morphol- and Nixon 1989b; Nixon and Crepet 1989). The ogy, other potentially diagnostic features to pollen of Quercus and the castaneoid genera are distinguish Notholithocarpus from Lithocarpus s. diagnostic in shape and exine sculpture. The s. are foliar, namely the lack ofan acuminate tip, pollen grains of Quercus are relatively large (27.5 sclerophyllous leaves bearing multiradiate tri- X 25.4 |Lim), subspheroidal, with a granularexine; chomes (Jones 1986, see Type 10), unbranched the grains of the castaneoid genera are smaller secondary veins that extend to the margin, and (18.0 X 10 |am), prolate, with a smooth to striate the potential for serrate margins in Notholitho- exine (Crepet and Daghlian 1980). Pollen varia- carpus, although some species ofLithocarpus s. s. tion among and within the castaneoid genera is also bear slightly toothed margins. Our studies mostly limited to size, but with a trend from more also included a comprehensive examination of prolate grains in Chrysolepis, Castanea, and literature-based reports on inflorescence structure, Lithocarpus s. s., to less prolate in Castanopsis morphology of staminate and pistillate flowers. Fig. 2. Pollen of select castaneoid taxa. A & B. Notholithocarpus densijlorus {Stone 3034); C & D. Chysolepis chrysophylla (I. Olmsted259)\ E & F. Lithoearpiis dealhatus {Manos 1288); G & H. Castcmopsis iiidica {Memosand Zhou 1426); Scales: A, C, E, and G = 5 ^im; B, D, F, and H = 2 [im. 2008] MANOS ET AL.: PHYLOGENY AND TAXONOMY OF PALEOENDEMIC FAGACEAE 187 Notholithocarpus (#) E ® Quercus 'Castanea Castanopsis Lithocarpus Chrysolepis <Trigonobalanus Formanodendron Colombobalanus Fagus Fig. 3. Summary ofthe phylogeny ofFagaceae with a set ofdiagrams and images ofrepresentative cupule/fruit types. Cupule valvesare indicated with straight lines and valvelesscupules with largecircles. Fruits aredrawn with discs or triangles, and aborted flower position with small circles. For example, the unifloral cupule/fruit in Notholithocarpus is shown with a disc in a large circle. Arrows indicate observed transformations within that clade orwithintheinflorescence. Photographsaretakenfromthefollowingspecies: Notholithocarpusdensiflorus: Quercus coccinea\ Castanea crenata; Castanopsis tribuloides (left) and Castanopsisfissa (right); Chysolepis chrysophylla: Lithocarpusfenestratus(left)and Lithocarpusrotunclatus(right); Colombobalanusexcelsa(left)andFornumoclenclron doichangensis (right); Fagus grcmdifolia. wood anatomy, and cupule ornamentation among evergreen taxa known to have dispersed through castaneoids (Carlquist 1984; Kaul and Abbe 1984; the North Hemisphere via Beringian and North Kaul 1987; also see http://insidewood.lib.ncsu.edu/ Atlantic Land Bridges (Tiffney and Manchester search/), but no clear differences were detected in 2001). these features. The phylogenetic position of CA-FP casta- Taxonomic Treatment neoids as highly divergent members of two different major Fagaceae lineages reinforces the Tanbark oak is a well-known element of the concept that these paleoendemic taxa are rem- CA-FP and is a major focus ofresearch into the nants of an ancient and formerly widespread spread of sudden oak death (SOD; Rizzo et al. broadleafevergreen flora, which persists today in 2005). Ourresultsclarify its phylogenetic position the Indochinese tropics. Additional evidence for in the Fagaceae and its affinities with temperate this is based on the appreciable level of fossil oaks and tropical chestnuts. The fact that it is diversity among likely thermophihc castaneoids more closely related to the victims of the in North America (Crepet 1989; Crepet and devastating chestnut blight, Castanea spp., than Daghlian 1980; Crepet and Nixon 1989a; Man- toitsmistakencongenerics, thestoneoaks, should chester 1994; Mindell et al. 2007). The macrofos- provide insight into the evolutionary dynamics of sil record also indicates the minimum divergence fungal infection and resistance. Here, we place time between Chrysolepis and Lithocarpus s.s. to tanbark oak into a newly recognized genus, be at least 40 mya based on fossils unambigu- Notholithocarpus, which reflects its false or ously assigned to Lithocarpus s.s. (Kvacek and convergent similarity to Asian Lithocarpus s.s. Walther 1989). This scenario suggests that the We make this new combination without subfa- CA-FP castaneoids were established in the early milial designation, as further work is required to Tertiary, which agrees with other broadleaf produce a valid nomenclature at that level. ) MADRONO 188 [Vol. 55 NothoUthocarpus Manos, Cannon & S. Oh, gen. Cannon, C. H. and P. S. Manos. 2000. The Bornean nov. TYPE: N. densiflorus (Hook. & Arn.) LitlwcarpusBl. section Synaedrys(Lindley) Barnett Manos, Cannon & S. Oh. (Fagaceae): discussion of its circumscription and A Lithocarpo foliis sclerophyllibus trichomatibus description of a new species. Botanical Journal of the Linnaean Society 133:343-357. multiradiatis et polline subprolato (sphaeroideo AND 2001. Combining and comparing differt. morphometric s.hape descriptors with a molecular Notholithocarpus densiflorus (Hook. & Arn.) phylogeny: the case of fruit type evolution in Manos, Cannon & S. Oh, comb. nov. Basionym: Bornean Litliocarpus (Fagaceae). Systematic Biol- Quercus densiflora Hook. & Arn., Bot. Voy. ogy 50:1-21. DB.eeDchoeuyg,laps.s3.9n1.,(1h8o4l0o.tyTpeY:PEK!:, iUsSoAty,pe:CaGliHf!o)rnia, Carlwtqrouaoicdsh:eta,sriygSn.eilfe1im9ce8an4nt.cse.VaeAnslsdieslroelg1ar0to:iu5op0ni5sh-ni5gp25it.no diimcpoetryfloerdaotne For intraspecific taxonomy, we follow other Crepet, W. L. 1989. History and implications of the treatments (Tucker 1993; Nixon 1997). early North American fossil record of Fagaceae. Notholithocarpus densiflorus var. densiflorus Pp. 45-66 in P. R. Crane and S. Blackmore (eds.). Evolution, systematics and fossil history of the Notholithocarpus densiflorus var. echinoides (R. Hamamelidae.Clarendon,Oxford,UnitedKingdom. Br. ter) Manos, Cannon & S. Oh, comb. nov. AND C. P. Daghlian. 1980. Castaneoid Basionym: Quercus echinoides R. Br. ter, Ann. inflorescences from the Middle Eocene ofTennes- Mag. Nat. Hist., 4. 7:251. 1871. TYPE: USA, see and the diagnostic value of pollen (at the Oregon, Canon Creek, Siskiyou Mountains, subfamily level) in Fagaceae. American Journal of Botany 67:739-757. BrTohwen g2e5n0us(HnoalomteypPeasKan,iai,soatylpoec:alK!n).ame for one evidAeNncDe Ko.fCF.agNaicxeoaen:. p1h9y8l9oag.enEeatrliicesatnmdegbaifoogsesiol- of the species in Java (Rehder 1916), also was graphic implications. American Journal ofBotany applied to tanbark oak byOrsted (1866). In order 76:842-855. to preserve its usage within the synonomy of AND 1989b. Extinct transitional Faga- . Lithocarpus, we designate a type for the genus ceae from the Oligocene and their phylogenetic name Pasania by using one of the species implications. American Journal of Botany originally listed by both Miquel (1857: Quercus 76:1493-1505. subgenus Pasania) and Orsted (1866: Pasania Faegri, K. and J. Iversen. 1950. Text-book of subgenus Eupasania). modern pollen analysis. Ejnar Munksgaard, Co- penhagen, Denmark. Pasania (Miq.) Oerst., Vidensk. Meddel. Dansk Felsenstein, J. 1985. Confidence limits on phyloge- Naturhist. Foren. Kjobenhavn, 8:81, 1866. Ba- nies: an approach using the bootstrap. Evolution sionym: Quercussubgenus Pasania Miq., Fl. Ned. 39:783-791. Ind. Vol. 1(1): 848. 1856. TYPE: Pasania sun- FoRMAN, L. L. 1966a. On the evolution ofcupules in daica (Blume) Oerst., {Quercus sundaica Blume) the Fagaceae. Kew Bulletin 18:385-^19. (lectotype designated here). . 1966b. Generic delimitation in the Castaneoi- deae (Fagaceae). Kew Bulletin 18:421-426. Acknowledgments Hjelmquist, H. 1948. Studies on the floral morphol- ogy and phylogeny of the Amentiferae. Botanical We are grateful to Don Stone and Leslie Eibest for Notiser 2(Suppl.):l-171. advice on acetolysis and assistance with the SEM Hooker, W. J. and G. A. W. Arnott. 1840. The images and to Elizabeth Wheeler for her insights on botany of Captain Beechey's voyage. Henry G. woodanatomy. Bill Buck,JonShaw,AlanWhittemore, Bohn, London, United Kingdom. Erin Tripp, and Robert Wilbur helped with the InsideWood. 2004-onwards. Published on the Inter- taxonomy, and Bill Buck added the Latin diagnosis. net. Available at: http://insidewood/lib.ncsu.edu/ Thanks to Erin Tripp, John L. Strother, and Bruce search Baldwin for improving the manuscript. This research Jones, J. H. 1986. Evolution of the Fagaceae: the was supported by National Science Foundation grants implications of foliar features. Annals of the DEB-9707945 to P. S. Manos and DEB-0445193 to P. Missouri Botanical Garden 73:228-275. S. Manos and S. Oh, and the National Evolutionary Kaul, R. B. 1987. Reproductive structure of Litho- Synthesis Center Northern Hemisphere Phytogeogra- carpus sensu lato (Fagaceae): cymules and fruits. phy Working Group. Journal ofthe Arnold Arboretum 68:73-104. and E. C. Abbe. 1984. Inflorescence architec- Literature Cited ture and evolution in the Fagaceae. Journal ofthe Barbour, M. G. and R. A. Minnich. 2000. Califor- Arnold Arboretum 65:375^01. nian uplandsandwoodlands. Pp. 161-202 in M. G. KvACEK, Z. AND H. Walther. 1989. Paleobotanical Barbourand W. D. Billings(eds.).North American studiesin FagaceaeoftheEuropeanTertiary. Plant terrestrial vegetation, 2nd ed. Cambridge Univer- Systematics and Evolution 162:213-229. sity Press, New York, NY. Li, R.-Q., Z.-D. Chen, A.-M. Lu, D. E. Soltis, P. S. Camus, A. 1936-1954. Les chenes monographic du SoLTis, AND P. S. Manos. 2004. Phylogenetic DNA genre Quercus (et LitJwcarpus). Encyclopedic econ- relationships in Fagales based on sequences omique de sylviculture. Vol. 6-8. Academic des from threegenomes. InternationalJournalofPlant Sciences, Paris, France. Sciences 165:311-324. 2008] MANOS ET AL.: PHYLOGENY AND TAXONOMY OF PALEOENDEMIC FAGACEAE 189 Maddison, D. R. and W. P. Maddison. 2000. Rehder, a. 1916. Additional species. Pp. 2479 in L. MacClade 4: Analysis ofphylogeny and character H. Bailey (ed.). The standard cyclopedia of evolution. Sinauer, Sunderland, MA. horticulture. Vol. V. The MacMillan Company, Manos, p. S. and a. M. Stanford. 2001. The London, United Kingdom. biogeography of Fagaceae: tracking the Tertiary . Additional species. Pp. 3569 in L. H. Bailey history oftemperate and subtropical forests ofthe (ed.). The Standard cyclopedia of horticulture. Northern Hemisphere. International Journal of Vol. VI. The MacMillan Company, London, Unit- Plant Sciences 162:S77-S93. ed Kingdom. and K. p. Steele. 1997. Phylogenetic analyses Rizzo, D. M., M. Garbelotto, and E. M. Hansen. of 'higher' Hamamelididae based on plastid se- 2005. Phytophthora ramorum: integrative research quence data. American Journal of Botany and management of an emerging pathogen in 84:1407-1419. California and Oregon forests. Annual Review of Z. Zhou, and C. H. Cannon. 2001. System- Phytopathology 43:309-335. , atics of Fagaceae: phylogenetic tests of reproduc- Shimodaira, H. and M. Hasegawa. 1999. Multiple tive trait evolution. International Journal ofPlant comparisonsoflog-likelihoods with applicationsto Sciences 162:1361-1379. phylogenetic inference. Molecular Biology and MacDonald, a. D. 1979. Inception of the cupule of Evolution 16:1114-1116. Quercus macrocarpa and Fagus grandifolia. Cana- Stebbins, G. L. and J. Major. 1965. Endemism and dian Journal ofBotany 57:1777-1782.^ speciation in the California Flora. Ecological Manchester, S. R. 1994. Fruits and seeds of the Monograph 35:1-35. middleEoceneNut Beds Flora, Clarno Formation, Swofford, D. L. 2002. PAUP* Phylogenetic Analysis Oregon. Palaeontographica Americana 58:1-205. Using Parsimony (* and other methods), version MiQUEL, F. A. W. 1857. Cupuliferae. Pp. 843-871 in 4.0. Sinauer Associates, Sunderland, MA. Flora van nederlandsch Indie. Van de Post, , G. K. Olsen, p. J. Waddell, and D. M. Amsterdam, the Netherlands. HILLIS. 1996. Phylogenetic inference. Pp. 407-514 MiNDELL, R. A., R. A. Stockey, and G. Beard. in D. M. Hillis, C. Moritz, and B. K. Mable (eds.). 2007. Cascadiacarpa spinosa gen. et sp. nov. Molecular systematics. SinauerAssociates, Sunder- (Fagaceae): castaneoid fruits from the Eocene of land, MA. Vancouver Island, Canada. American Journal of TiFFNEY, B. H. AND S. R. MANCHESTER. 2001. Theusc Botany 94:351-361. of geological and paleontological evidence in Nixon, K. C. 1997. Fagaceae. Pp. 436-506 in Flora of evaluating plant phylogenetic hypotheses in the North America Editorial Committee (eds.). Flora Northern HemisphereTertiary. International Jour- of North America north of Mexico. Vol. 3. nal ofPlant Sciences 162(Suppl.):S3-S17. Magnoliophyta: Magnoliidae and Hamamelidae. Tucker, J. M. 1993. Fagaceae. Pp. 657-665 in J. C. Oxford University Press, New York, NY. Hickman (ed.). The Jepson manual. University of AND J. M. Carpenter. 1996. On consensus, California Press, Berkeley, CA. collapsibility, andcladeconcordance. Cladistics 12: Wang, P-L. and K-L. Chang. 1989. A study on 305-321. pohen morphology and ultrastructure ofsubfamily AND W. L. Crepet. 1989. Trigonobalanus Castaneoideae (Fagaceae) in China. Acta Phyto- (Fagaceae): taxonomic status and phylogenetic taxonomica Sinica 3:205-214. relationships. American Journal of Botany 6: ZwiCKL, D. J. 2006. Genetic algorithm approaches for 828-841. the phylogenetic analysis of large biological se- Oh, S. and p. S. Manos. 2008. Molecular phyloge- quence datasets under the maximum likelihood netics and cupuleevolution in Fagaceae as inferred criterion. Ph.D. dissertation. The University of from nuclear CRABS CLAW sequences. Taxon Texas, Austin, TX. Available at: http://www.bio. 57:434-451. utexas.edu/faculty/antisense/garli/Garli.html [Ac- Okamoto, M. 1989. A comparative study of the cessed 10 May 2007]. ontogenetic development of the cupules in Casta- nea and Lithocarpus (Fagaceae). Plant Systematics Appendix OlmsatnedaEdv,olRu.tiGo.na1n6d8:J7.-1A8..Sweere. 1994. Combining nDumNbAervsoufocrhetraxianfuosremdatiniotnheanmdoI.lGeceunlBaarnpkhyalcocgeesnseitoinc data in phylogenetic systematics: an empirical study. Each entry includes species, locality, voucher approach using three molecular data sets in the specimen, and GenBank accessionnumbers(ITS; CRC\ Solanaceae. Systematic Biology 43:467^81. Orsted, a. S. 1866. Bidrag til Egelaegtens systematik. trnKlmatK\ atpB-rbcL\ ncI/iFaccession). Videnskabelige Meddelelser fra Dansk Naturhis- Castanea mollissima Blume; USA, New York, torisk Forening i Kjobenhavn 8:11-88. Tompkins Co., Cornell University Plantations; Manos Posada, D. and K. A. Crandall. 1998. MODELT- 1038 (BH); ITS-AY040396; C7?C EU189752; tniKJ EST: testing the model of DNA substitution. matK-¥iU5050\ nd/iF-FJ1^5073; Connecticut, Con- Bioinformatics 14:817-818. necticut Agricultural Research Station; StanfordRlT15 Prantl, K. 1888. Fagaceae. Pp. 47-58 inA. Englerand (UNC-CH); atpB-rbcL-AY0A2A53. K. Prantl(eds.). Dienatiiralichen Pflanzenfamilien. Castanopsis argyrophylla King ex Hook.f.; China, Division III, Vol. 1, Wilhelm Engelmann, Leipzig, Yunnan, Menglian; Manos and Zhou 1402 (DUKE); Germany. AY040376; EU189758; FJ185051; FJ185059; FJ185074. Raven, P. H. and D. I. Axelrod. 1978. Origin and Castanopsis carlesii (Hemsl.) Hayata; China, Yun- relationships of the Cahfornia flora. University of nan,Jingu; ManosandZhou 1382 (DUKE); AY040372; CaHfornia Publications in Botany 72:1-134. EU189760; FJ185052; FJ185060; FJ185075. MADRONO 190 [Vol. 55 Castcmopsisfissa(Champ, exBenth.) Rehder& E. H. echinoides (R. Br.) Abrams); USA; California, Ne- Wilson; China, Yunnan, Simao; Memos andZhou 1396 vada Co., Washington; Manos and Tucker 922 (DUKE); AY040392; EU189763; FJ185053; FJ185061; (BH); AY040370, EU189783; FJ185047; FJ185067; FJ185076. FJ185081. Chrysolepis chrysophylla (Douglas ex Hook.) Quercus myrsinifoHa Blume; USA, Georgia, USDA Hjelmq.; USA, Oregon, Benton Co., St. Mary's Peak; Coastal Research Station. Savannah; Memos s.n. Memoss.n. (DUKE); AF389087; EU189770; FJ185045; (BH); ITS-AF098414; California, Yolo Co., Shields FJ185062; FJ185077. Grove Arboretum; no voucher; C7?C-EU189824; trnKI Colombohahmus excelsa (Lozano, Hdz-C. & Henao) matK- FJ185048; atpB-rbcL- FJ185068; ndhF^ Nixon & Crepet; Colombia, Virolin; Nixon 4655 (BH); FJ185084. AF098412; EU189772; AY042456+AY040492; Quercus rohur L.; USA, New York, Tompkins Co., FJ185063; FJ185078. Cornell Univ. Campus; Manos s.n. (BH); AF098424; Fagusgrcmdifolia Ehrh.; USA, NewYork, Tompkins EU189832; FJ185056; AY042505; FJ185085. Co.; Manos 114 (BH); AY040509; EU189851; Quercus rubra L.; USA, New York, Tompkins Co., U92861+AY042400; AY042412; FJ185079. Cornell Univ. Campus; Manos s.n. (BH); AF098418; Fornumodendron doicJumgensis (A. Camus) Nixon & EU189836; FJ185057; FJ185069; not determined. Crepet; China, Yunnan, Menglian; Manos and Zhou Quercus suber L.; USA, California, Santa Barbara 1400 (DUKE); AY040452; EU189773; FJ185046; Co., Orella St.; Manos 423 (BH); AF098434; FJ185064; FJ185080. EU189843; FJ185049; FJ185070; FJ185086. Lithocarpus pachvlepis A. Camus; China, Yunnan, Quercus tonientella Engelm. USA, California. Da Wei Shan; Manos and Zhou 1451 (DUKE); Santa Barbara Co. Santa Cruz Island; Memos 983 AY040441; EU189792; FJ185054; FJ185065; FJ185082. (BH); AF098437; EU189845; FJ185058; FJ185071; Lithocarpusxylocarpus (Kurz) Markgr.; China, Yun- FJ185087. nan, Da Wei Shan; Manos and Zhou 1463 (DUKE); Trigemobalanus verticil/ata Forman; U.K., Scotland, AY040426; EU189801; FJ185055; FJ185066; FJ185083. Edinburgh, Royal Botanical Gardens; RBG 1967-421; NotJioUtJwcarpus densifloras var. echinoides (R. Br. AF098413; EU189847; U92866+AY042455; FJ185072; ter) Manos & S. Oh {Lithocarpus densiflorus var. FJ185088.
