Organic molecules with functional groups containing oxygen PDF
Preview Organic molecules with functional groups containing oxygen
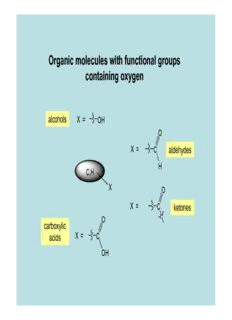
Organic molecules with functional groups containing oxygen alcohols X = OH O X = C aldehydes H C,H X O X = C ketones O carboxylic acids X = C OH Key Skills 1. Dealing with structures (Bruice 1.4) We need to understand the following concepts: • Valency: the number of bonds that an atom must have eg carbon: 4; hydrogen: 1; oxygen: 2 H H • Bond Concept: a pair of electrons H C C H H H C C C C To break a bond, the To make a bond, a pair electron pair has to move of electrons has to away from the space move into the space between the atoms between the atoms • Drawing structures: we can represent a molecule in a variety of ways Example: ethanol, C H O 2 6 CH 3 CH 2 H H CH CH OH 3 2 C C OH H C C OH OH H CCH OH 3 2 H H Never!! - A bit long Correct – Compact – unless you mean winded – but useful if it is the 1,1,2,2- good if you space is an standard tetramethylpropanol! want to use issue for large the structure in molecules a mechanism The ability to “read” and draw structural formulae is an absolutely essential skill! Problem How many hydrogens are on each of the carbons indicated below? OH OH OH OH A very, very, very, common mistake! ≠ • What is the molecular formula of each of the above molecules? • How many hydrogens are on each of the carbons? • What is the name of each compound? • Is there any other way of drawing the first structure? 2. Dealing with mechanisms (Bruice 1.18, 3.6) • A mechanism is a description, in terms of the electrons involved, of how the reactant molecule(s) changes into the product molecule(s). “Curly arrows” are used to show how the electrons move during the change. • Most reactions involve intermediates, the nature of which determine the type of mechanism involved reactant intermediate product • Reactions can involve a neutral intermediate with an unpaired electron (a radical) or a charged intermediate (a cation (+) or an anion (-)) Example: a reaction mechanism involving charged intermediates H H H ≡ H H H H H H SO 2 4 + H O 2 H C C O H H C C O C C + H H H H H H H H H • The arrows are double headed in this case, indicating the movement of a pair of electrons • The arrows begin at a definite pair of electrons - a bond or a lone pair – and move towards a positive charge • If they move into the space between two atoms, a bond is formed • If they move out of the space between two atoms, a bond is broken • If a new bond is formed with a neutral atom, another bond involving that atom has to be broken • If an atom gains an electron, it acquires a negative charge; if an atom loses an electron, it acquires a positive charge The Chemistry of Alcohols Functional Group General Alcohols δ− R O δ+ C,H R H functional group δ− Key Point: alcohols and water contain O δ+ the same functional group (FG) H H Famous Alcohols 1. Ethanol H H • Structure H C C O H or H CCH OH or 3 2 OH H H • World Production (2006): 51 gigalitres (5.1 x 1010litres) – 69% from the US/Brazil • Methods of Production (a) Hydration of ethene: production of ethanol for use as an industrial feedstock H H H PO on 3 4 crude charcoal gas phase oil C C + H2O H3CCH2OH reaction 300°C H H ethanol produced in this way is a petrochemical: non-renewable/not sustainable (b) Fermentation yeast C H O H CCH OH + 2CO 6 12 6 3 2 2 no O 2 fermentable sugars such as glucose, fructose or sucrose (C H O ) 12 22 11 H SO malting - 2 4 involves the enzyme corn (starch) sugar amylase (US): cane production of (Brazil) barley: ethanol a a fuel production of ethanol as a beverage (c) Cellulosic ethanol Cellulose is a glucose polymer which makes up 38% of all plant matter but which cannot be fermented directly enzymic hydrolysis cellulose glucose cellulase fermentable sugar materials such as straw, sawdust, bagasse (residue after extraction of sugars from sugar cane), switchgrass (an “energy crop”) (d) Bioethanol production in Ireland The fluid left when the solids are removed from the milk during the making of cheese is called whey and contains fermentable sugars. This is currently the source of all bioethanol produced in Ireland. However the amount of bioethanol available from this source would not be sufficient to satisfy the demand for it as a fuel.
Description: