NESTING ECOLOGY OF LESSER PRAIRIE-CHICKENS IN SAND SAGEBRUSH PRAIRIE OF SOUTHWESTERN KANSAS PDF
Preview NESTING ECOLOGY OF LESSER PRAIRIE-CHICKENS IN SAND SAGEBRUSH PRAIRIE OF SOUTHWESTERN KANSAS
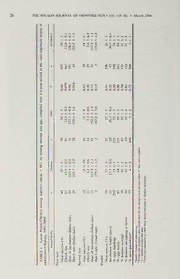
) The Wilson Journal ofOrnithology 118(1):23—35, 2006 6 NESTING ECOLOGY OF LESSER PRAIRIE-CHICKENS IN SAND SAGEBRUSH PRAIRIE OF SOUTHWESTERN KANSAS JAMES C. PITMAN,'-4-8 CHRISTIAN A. HAGEN,1-5 BRENT E. JAMISON,1- ROBERT J. ROBEL,1 THOMAS M. LOUGHIN,2 AND ROGER D. APPLEGATE57 — ABSTRACT. Despite the fact that the Lesser Prairie-Chicken (Tympanuchuspallidicinctus) is a species of conservationconcern, little isknownabout itsnestingecology,particularly insand sagebrush(.Artemisiafilifolia) habitats. To find and monitor nests, we captured and equipped 227 female Lesser Prairie-Chickens with trans- mitters (87 yearlings, 117 adults, and 23 ofunknown age) from 1997 to 2002 in southwestern Kansas. Apparent nest success was similar for yearlings (31%, n = 74) and adults (27%, n = 97) but differed marginally (P = 0.090) between first nests (29%) and renests (14%). An estimated 31% of females that were unsuccessful in their first nesting attempt initiated a second nest. The probability thata female would initiate a second nestafter failure of the initial attempt was negatively influenced by the day of incubation on which the initial attempt failed. Over 95% of all nests were initiated and completed between 5 May and 2 July. The primary cause of nest failure was predation by coyotes (Canis latrans) and gopher snakes (Pituophismelanoleucus). Meanclutch size, egg fertility, hatching success, nesting and renesting frequency, and incidence of interspecific parasitism were all similar across years and between yearlings and adults. Distances between nest sites were used as an index to nest-site fidelity between first nests and renests and for across-year nesting attempts. Mean distances betweenfirstnestsandrenests were similarforyearlings (1,071 m) andadults (1,182m).Meandistancebetween nests constructed by the same female in subsequent years (918 m) did not differbetween age classes orsuccess ofthe first year’s nest. Most females (80%) nested closer to a lek other than the lek where they were captured. Received24 January 2005, accepted21 September2005. Range-wide, Lesser Prairie-Chickens Tym- Prairie-Chickens currently occupy 31 of 39 ( panuchus pallidicinctus have declined by an counties believed to compose their historical estimated 97% since the 1800s (Crawford distribution in Kansas, but counts of leks and 1980, Taylor and Guthery 1980). In Kansas, individual birds suggest that Lesser Prairie- Lesser Prairie-Chickens are—most abundant in Chickens have experienced significant de- the western part ofthe state south ofthe Ar- clines since 1964 (Jensen et al. 2000). kansas River in mixed and shortgrass prairie The mechanisms responsible for Lesser dominated by sand sagebrush {Artemisiafili- Prairie-Chicken population declines have not folia). They also occur in mixed grass prairie been identified; however, aspects of nesting north of the Arkansas River, but this habitat ecology could be influential (Peterson and Sil- is generally devoid of sand sagebrush. Lesser vy 1996, Wisdom and Mills 1997). Thus, identifying age-specific variation in nesting variables is important to understanding a spe- KS1 D6i6v5.06o,fUBSiAol.ogy, Kansas State Univ., Manhattan, cies’ demography or life-history strategy (Pat- 2Dept, ofStatistics, Kansas State Univ., Manhattan, ten et al. 2005). Most research on LesserPrai- KS 66506, USA. rie-Chicken nesting ecology has been con- 3Survey and Research Office, Kansas Dept, of ducted in sand shinnery oak {Quercus havar- Wildlife and Parks, P.O. Box 1525, Emporia, KS dii) habitats in New Mexico and Texas (Davis 66801, USA. et al. 1979, Haukos and Broda 1989, Riley et sas4CDuerprte,ntofadWdirledslsi:feSuarnvdeyPaarnkds,ReP.sOe.arBcohxOf1f5ic2e5,,KEamn-- al. 1992). The objectives of our study were to poria, KS 66801, USA. provide baseline information on age-specific 5Current address: Oregon Dept, of Fish and Wild- variation in nesting ecology, record fidelity to life, 61374 Parrell Rd., Bend, OR 97702, USA. previous nest sites (within-year renests and 6Current address: Missouri Dept, of Conservation, across-year attempts), and document nest-site P.O. Box 368, Clinton, MO 64735, USA. locations relative to leks of Lesser Prairie- 7Current address: Tennessee Wildlife Resources Chickens in sand sagebrush prairie of south- 4A0g7e4n7c,y,NasEhlvliilnlget,oTnNAg3r7i2c0u4l,tuUrSalA.Center, P.O. Box western Kansas. We examined annual varia- 8Corresponding author; e-mail: tion and the effects ofage on reproductive pa- [email protected] rameters and nest-site placement. 23 24 THE WILSON JOURNAL OF ORNITHOLOGY • Vol. 118, No. 1, March 2006 METHODS fixed-wing aircraft. We monitored females Study area.—From 1997 to 2002, we stud- that moved off our study area two to three times per week throughout the nesting season. ied Lesser Prairie-Chickens inhabiting sand Using a hand-held antenna, we found nests sagebrush habitat south ofthe Arkansas River by approaching transmitter-equipped females in Finney County, Kansas (37° 52' N, 100° 59' W). We initiated field work on a 7,700-ha w>h3encotnhseeicrultoicvaetidoanyss.hadIfrtehmeaifneemdaluencwhaasngiend- area in 1997 and on a nearby 5,600-ha area in 2000; we continued work on both areas cubating, she was flushed so the eggs could tinhrbooutghh saruemasm;ersa2n0d02s.agVeegbertuasthiownaswatshesimmiolsatr bspeecciofuicntepdaraasnidtitshme c(lHuatgcehnexeatmialn.ed20f0o2r).intWere- marked nest locations with flags (1997) or conspicuous vegetation present and was inter- m transmitters (1998-1999) at a distance of 5 spersed with grasses, including little bluestem from the nest bowl (Jamison 2000), or we re- Schizachyrium scoparium), needle-and- ( corded locations with a global positioning sys- thread Stipa comata), sand lovegrass Era- ( (. tem (2000-2002). Nest sites were not visited grostis trichodes sixweeks fescue Vulpia ), ( again until the female departed the site with a octoflora), blue grama (Bouteloua gracilis), brood or until the nest was depredated or sand dropseed Sporobolus cryptandrus ( ), abandoned. This technique allowed us to es- sideoats grama (B. curtipendula), and western timate apparent nest success only. Because we wheatgrass (Agropyron smithii). The most did not determine nest status throughout in- common forb species were Russian thistle cubation, we did not estimate daily survival (Salsola kali), western ragweed (Ambrosia of eggs or nests according to the Mayfield psilostachya), sand lily (Leucocrinummontan- method (Mayfield 1975). um), and common sunflower Helianthus an- ( After the departure of each nesting female, nuus). Each study area was bounded almost we classified nest fate as successful (produced entirely by center-pivot irrigated cropland and at least one chick), unsuccessful, or aban- grazed seasonally by livestock. Annual pre- doned. Beginning in 2000, we opened un- cipitation averaged 50 cm (U.S. Department hatched eggs to determine whether embryos of Commerce 2003) and ranged from 42 cm had developed. Ifthe nest was depredated, we (2000) to 59 cm (1997) during our st—udy. systematically searched the area within a 10- Locating and monitoring nests. Using m radius for tracks, scat, or eggshell frag- walk-in funnel traps, we captured female ments to help determine the predator’s identity LMeasrscerh Ptrharioruige-hChmiickde-nAsproinl l(eHkasukforsometmiadl-. (SaSrtgateiasntticeatlala.na1l9y9s8e)s..—We recorded clutch 1990). Except in 1997 (when age was not de- size and estimated the start of incubation for termined), we classified captured birds as yearling and adult nests. We defined the start yearlings (—10 months of age) or adults (>21 of incubation as the first day on which we m(Coonptehlsinof196a3g)e.) Wbey eeqxuaimpipneidngbirtdhse wpirtihma1r1i-egs dloectaetcitoends—notcyphiacnaglleys, i3n-t5hedfaeymsalbee’fsorteeleamentersyt = necklace-style transmitters (life expectancy was located. We estimated the initiation date AVM 6-12 months; models from Instrument of each nest by backdating from the start of Company, Colfax, California; Advanced Te- incubation by 1 day for each egg in the clutch lemetry Systems, Isanti, Minnesota; and Ho- (Coats 1955). We also calculated apparent lohil Systems, Carp, Ontario) and released nest success (the proportion ofall known nests them on-site immediately after capture. Each producing at least one chick X 100), hatching day, we determined locations of transmitter- success, egg fertility, percentage of females equipped birds by triangulating bearings col- attempting a nest, percentage of females re- lected from a truck-mounted, null-peak telem- nesting,—and the incidence ofinterspecific par- etry system. Bird locations were determined asitism separately for yearlings and adults. until transmitter failure, emigration from the We defined hatching success as the number of primary study areas, or bird death. When birds eggs hatched divided by initial clutch size emigrated from our study area, we re-located (Westemeier et al. 1998b). We defined percent them by extensive ground searches or from fertility as the number of eggs hatching or Pitman et al. • NESTING ECOLOGY OF LESSER PRAIRIE-CHICKENS 25 containing a developed embryo divided by the cluded failed first nest attempts recorded from total number of eggs in the nest bowl at the 1997 to 2002. We used the same model pro- time of hatching. We estimated incubation cedures previously described to fit three ofour length as the time (days) between the start of a priori models that included the main effects incubation and the date when a female left the (1) clutch size and (2) day of incubation on nest with a brood (as determined from telem- which the initial attempt failed. etry locations). We estimated nesting frequen- We calculated distances between first nests cy as the percentage offemales that attempted and renests, nesting attempts in multiple a nest. Females that did not attempt a nest and years, and distances from nest sites to the lek died before 31 May were excluded from our of capture and the nearest lek. We used anal- estimate of nesting frequency. Because we ysis of variance (ANOVA) to determine documented some first nesting attempts after whether year or age class influenced the dis- 31 May, it was uncertain whether birds dying tance between an initial nest site and the re- prior to this date would have subsequently at- nest location and the affinity of nesting fe- tempted a nest. Interspecific parasitism was males to lek sites (capture lek and nearestlek). reported as the percentage ofnests containing We also used ANOVA to determine whether eggs ofboth Lesser Prairie-Chickens and oth- age class or success of the first-year nest af- er bird species. Interspecific nest parasitism fected distance between nest sites in subse- was previously described for the 1997 to 1999 quent years. For these analyses, we excluded field seasons (Hagen et al. 2002); here, we all data from 1997 because we did not identify summarize all records of parasitism from age class that year; however, we included 1997 to 2002. The percentage of females at- pooled age-class data from 1997 in the data tempting to renest was estimated as the per- tables to provide an overview of nesting pa- centage of females known to have incubated rameters for the duration of our study. We in- and lost a first clutch and that subsequently terpreted simple effects with two-sample t- incubated a second. Because of some small tests when significant interactions were found expected cell counts, we used a Fisher’s exact (Zar 1999). We considered all differences sig- test for all comparisons (Agresti 1996). In ad- nificant when P < 0.05 and marginally sig- dition, we used two-tailed f-tests for unequal nificant when 0.05 < P < 0.10. We report variances (Zar 1999) to compare clutch size, parameter estimates and means as ± SE (or incubation date, hatch date, and incubation SD as noted). length between yearlings and adults. We used logistic regression to assess the re- RESULTS — lationship between the likelihood of renesting Nesting ecology. We captured 227 female and (1) age class, (2) clutch size of the initial Lesser Prairie-Chickens and fitted them with nest attempt, and (3) day into incubation when transmitters (87 yearlings, 117 adults, and 23 the initial attempt failed. We excluded data of unknown age). We found 209 nests (77 from 1997 because we did not identify age yearling, 103 adult, and 29 unknown-age). class of birds that year. Initially, we fit seven The percentage of females initiating a nest a priori models to data associated with 59 was similar (P = 0.50) for yearlings (94%) failed first nest attempts recorded from 1998 and adults (92%; Table 1). We determined fate to 2002. We considered all four additive mod- for 196 of209 (94%) nests; apparent nest suc- els and main effect models for each of the cess was 26 ± 3% (51 of 196). The remaining three independent terms. We used the mini- nests were either abandoned (2%, n = 5) or mization ofAkaike’s Information Criterionfor success could not be determined from evi- s(mBaulrlnhsaammplaendsizAensde(rAsIoCnc.) t1o99r8a)n.kAtlhle mmooddeellss dNeesntcesurcecmeasisnidnigd natotthdeifnfeesrtacsirtoess(4y%e,arns (=x28)—. pwehteirneg AmoAdIeClcs<(B2urwnerheamcoannsdidAenrdeedrtsoonbe19c9o8m)-. f6o.r95f,irdstfn=es5t,sP(P==0.02.26)0)ororbertewneesetnsa(gPe=cl0a.s8s2e;s Because age class was not included in any of Table 1). An estimated 31% ofall females that the competing models (all AAICc > 2), we were unsuccessful in their first nesting attempt excluded this variable and developed models initiated a second nest, and this percentage did using an expanded data set (n = 69) that in- not differ (P = 0.85) between yearlings and ' 26 THE WILSON JOURNAL OF ORNITHOLOGY Vol. 118, No. 1, March 2006 of prairie — o o c—n o c—n cn o — NO cn CN CN O+N1 o+1 i+n1 0+01 S—+t1 q+1 c+n1 o+1 N+O1 +1 +1 +1 C+N1 _+_1 c+n1 sagebrush CN r—-i C0N0 cinn icnn >n CN CNNO ON ON cn sand — — m o — the ir-n no TLTf; cn ocn> cn >o n—o cTtn h m-H nh—o C-—NH OOn in LT, <N period ON o o o o 6-year Oto-* OoN 3oor C0oN0 con cron- co—n >dn Co>Nn ion OoN oin 0o>0n CrdN- a over CoN OoN q— oNO a—s o NoO compiled in 00 cn in cn CN cn NO CN _+1 +1 +1 +1 +1 +1 +1 o+1 +1 +1 +1 +1 +1 +1 +1 cn c—CnN cUn c—n cn CONN U Cr-N~ cNOn CrN- O>nn OCNN cn cn age, CN >n in NO CN and — M o M n attempt 0r0- oo rN-O ('Nt v—O — I—T) ON CN CcNn CcNn oo in o cn cn nesting excluded. by CoN OOn o 0— NoO CN >—n qd were »n CN CN in cn cn cn 00 CN S±E), ic+1nn 0—+01 is+j>-1 Nmr+O-1 i—+n1 0C+0N1 ac+sn1 n>+n1 c+n1 c+n1 f<+"n1 O'+'N1t O+N1 cc+n1n +1 3Ma1y CN in r» CN (mean before Bobwhites. died o — o " h o o h and statistics o o r" \hr n x h h in (N r, r, nest Northern CN a or nesting attempt Pheasants not did that Ring-necked Prairie-Chicken females age. nests.either nest; Lesser !•? PI3 ^§ LetgOf)26c0 s 5 ounknfownattaemptedffaiirlsetdpbarasiytized 1. o o 5M|<2u a8 £°g -a_O ^ cfl c/3 femalesthatonumfber were <U ^ o ^ -f TABLE ^ <u _U5 c2§/$ z« u= w| a3c g* s* ^ ^ e* IancludesbFemalescn= dNests Pitman etal. • NESTING ECOLOGY OF LESSER PRAIRIE-CHICKENS 27 — adults (Table 1). However, success of renests Renesting probability. The probability of (14%) was marginally less than success ofini- a Lesser Prairie-Chicken renesting was influ- tial nests (29%; X2 = 3.31, df = 1, P = 0.090). enced by both clutch size and the day of in- No females were known to have initiated a cubation on which the initial attempt failed. third nest in the same year. Mean hatch date An additive model including both terms was (all years combined) was 1 June for first nest- the highest-ranking (AAICc = 0.00; AICc = ing attempts and 22 June for renests (Fig. 1), 80.90), but the model including only date of with a mean incubation length of 26.7 days failure also had considerable support (AAIC (Table 1). More than 95% of all nests were = 1.48). The model including only clutch size( initiated and completed between 5 May and 2 was not supported (AAIC = 15.24). Females c July (Fig. 1). incubating initial nests later into incubation Mean clutch size did not differ between tended to have a lowerprobability ofrenesting yearlings and adults for either first nesting or (Gdate = -0.18, 95% Cl = -0.28 to -0.08; renesting attempts (Table 1). Mean clutch size Fig. 2). Females laying a larger clutch in the was 7.6 ± 0.4 eggs for renests, significantly initial nest attempt tended to be more likely less (f188 = 1 1.77, P < 0.001) than the mean to renest (Bclutch = 0.31); however, the magni- clutch size (12.0 ± 0.1 eggs) of first nests. tude of this effect was not clear because the Overall hatching success was 74 ± 2% and confidence interval overlapped zero (95% Cl did not differ between yearlings and adults. = —0.01 to 0.63). The odds of a female at- Likewise, egg fertility was similar between tempting to renest decreased by 16.2% with the two age classes, with 94 ± 1% of all eggs each day into incubation of the initial attempt containing a developed embryo (Table 1). and increased 20.2% with each one-egg in- Six of 209 (3%) Lesser Prairie-Chicken crease in clutch size (F—ig. 2). nests were parasitized by other bird species. Nest-site location. Between 1997 and Four of the six nests contained Lesser Prairie- 2002, we found 28 renests (Table 3). Distance Chicken and Ring-necked Pheasant (Phasi- between first nests and renests (1,271 m) was anus colchicus) eggs, and eggs ofboth species not influenced by age class (Flf23 = 1.69, P = hatched in two of these nests. One nest was 0.21) or year (F = 1.65, P = 0.21); there parasitized by a Northern Bobwhite (Colinus was no interacti4o>2n3 effect (F2i23 — 1.82, P = virginianus\ 10 prairie-chicken eggs and 1 0.19; 1998-2002 data). Similarly, the distance quail egg), and the remaining nest was para- between nests initiated by the same female in sitized by both Ring-necked Pheasant and subsequent years (mean = 918 m, n = 15; Northern Bobwhite (3 prairie-chicken eggs, 1 Table 3) was not influenced by age class (FU4 pheasant egg, and 1 quail egg). Both of these = 0.16, P = 0.70) or success of the first-year latter nests were d—epredated before hatching. nest (FU4 = 0.05, P = 0.82); there was no Nestpredators. Most (>80%) known pre- interaction effect (FU4 = 0.00, P = 0.98). dation events occurred >3 days after our ini- The distance from a nest to the nearest lek tial nest visit (mean = 10.2 days ± 6.9 SD). (mean = 691 m, n = 194; Table 4) was not We assigned predator species to 112 of 161 influenced by year (F4164 = 1.11, P = 0.36) (70%) unsuccessful Lesser Prairie-Chicken or age class (FU64 = 0.00, P = 0.99), nor was nests. Coyotes (Canis latrans) depredated the there an interaction effect (F4164 = 1.41, P = majority (64%) of the nests and were the pri- 0.23; 1998-2002 data). Of 184 nests, 147 mary cause of nest predation during most (80%) were located closer to a lek other than years (Table 2). Snakes were responsible for the lek where the female was last captured. the loss of 31% and 42% of the unsuccessful Ten nests (5%) were located >10 km from the Lesser Prairie-Chicken nests in 2001 and lek at which the incubating female was cap- 2002, respectively. Most of the snake preda- tured (median = 20.6 km, range = 10.6-56.5 tion was probably by Gopher snakes Pituo- km). The female nesting 56.5 km from her lek (. phis melanoleucus) because they were the of capture was successful in her nesting at- most observed snake species on our study ar- tempt. The distance from nest site to the lek eas. Other causes of nest loss included pre- where the female was captured (mean = 3,082 dation by ground squirrels (Spermophilus m, n = 184; Table 4) was not influenced by spp.) and trampling by cattle (Table 2). age class (FU58 = 0.12, P = 0.73) or year 28 THE WILSON JOURNAL OF ORNITHOLOGY • Vol. 118, No. 1, March 2006 nests of Percentage nests of Percentage Weekly interval FIG. 1. Percentage of Lesser Prairie-Chicken first nests (A) and renests (B) in southwestern Kansas that wereinitiated, incubated,depredated, andhatched, by weekly intervals, 1997-2002. Meandatesforeachvariable are listed at the top ofeach figure. Pitman et al. • NESTING ECOLOGY OF LESSER PRAIRIE-CHICKENS 29 TABLE 2. Probable causes of predation of Lesser Prairie-Chicken nests in the sand sagebrush prairie of southwestern Kansas, 1997-2002. Depredation(%) Predator (n1=99724) (n1=99812) in1=99920) in2=00404) in2=00136) in2=00226) inTo=tal1631) Coyote 71 100 70 34 22 27 45 Ground squirreP 4 0 0 11 0 0 4 Snakec 13 0 5 11 31 42 19 Cattle 0 0 5 2 3 0 2 Unknown 13 0 20 41 45 31 30 aPercentageofallnestsdestroyedbyeachpredator. bWedidnotdifferentiatebetweenthirteen-linedgroundsquirrelsandspottedgroundsquirrels. cGophersnakesappearedtobethemostabundantsnakespecies. (F4158 = 1.25 P = 0.29), and there was no ied substantially during the 6 years of our interaction effect (F4158 = 1.33, P = 0.26; study (range — 22.3-38.3 cm), we document- 1998-2002 data). ed little annual variation in Lesser Prairie- Chicken nesting activity. Our ability to detect DISCUSSION annual variation, however, may have been hin- Although rainfall during the primary 4- dered by relatively small sample sizes within month nesting period (April throughJuly) var- years, especially in the early years of the FIG. 2. Probability of Lesser Prairie-Chickens initiating renests after failure of the initial nest attempt in southwestern Kansas, 1997-2002. Probabilities are plotted for various clutch sizes (8, 10, 12, 14) and the day of incubation when the initial nest attempt failed. 30 THE WILSON JOURNAL OF ORNITHOLOGY • Vol. 118, No. 1, March 2006 TABLE 3. Evidence ofnest-site fidelity as shown by mean distances (m) between nests for Lesser Prairie- Chickens in southwestern Kansas, 1997-2002. Within- and across-yeardistances are presented by age class and nest fate. Within-year* Acrossyearsb’c Category n Distance SE n Distance SE Age class Yearling 11 1,071 327 6 1,170 599 Adult 13 1,182 263 9 750 365 Nest fated — — — Successful — 6 712 438 Unsuccessful 9 1,055 453 Totale 28 1.271 218 15 918 316 aDistancebetweenthefirstnestandtherenest. bFortwo females that initiated >1 nest within a year, the mean coordinates ofthose nests were used to calculate the distance to the nest site in subsequentyears. cNestsforonefemalewerelocatedinnon-consecutiveyears;allothernestswerelocatedinconsecutiveyears. dNestfatereferstofateoffirstnests. eAgeoffourfemaleswasundetermined. study. Additionall—y, we observed little age- tentiveness ofgrouse increases throughout the specific variation except that yearlings had laying period (Giesen and Braun 1979), we slightly smaller clutches and marginally later may have overestimated incubation length by hatch dates for first nest attempts than did misidentifying the start of incubation. How- adults. ever, the time required to hatch LesserPrairie- For all known nests, initiation began in ear- Chicken eggs in an incubator (24—26 days; ly May; peak hatching was 1 June for first Coats 1955, Sutton 1968) was only slightly nests and 22 June for renests (Fig. 1). Similar less than our estimate for eggs incubated by dates ofnest initiation (mid-April through late wild birds. May) and hatching (late May through mid- The success of all nests averaged 26% in June) have been reported from studies our study, substantially less than estimates throughout the species’ range (Giesen 1998, from New Mexico (42%) and Oklahoma Patten et al. 2005). Mean incubation length (40%; Patten et al. 2005), but similar to the was 26.7 days (this study). Because nest at- 28% reported by Giesen (1998) for 10 studies TABLE 4. Distances (m) between Lesser Prairie-Chicken nest sites and leks in southwestern Kansas, 1997- 2002. Nestsitetolekofcapture Nestsitetonearestlek Category n Median Mean ± SE n Median Mean ± SE Year 1997 25 1,528 1,647 ± 226 26 556 557 ± 52 1998 14 1,134 1,727 ± 529 14 577 546 ± 71 1999 24 2,357 2,317 + 332 25 726 701 ± 55 2000 56 1,282 2,874 -t- 1,006 56 675 742 ± 53 2001 37 1,396 3,241 ± 983 41 727 740 ± 54 2002 28 2,333 5,901 ± 1,366 32 631 703 ± 65 Age Yearling 68 1,893 3,580 ± 853 68 633 702 ± 48 Adult 91 1,258 3,104 ± 591 97 675 718 ± 32 Total 184a 1,427 3,082 4- 432 194b 632 691 ± 25 aIncludes25nestsoffemalesofunknownage. bIncludes29nestsoffemalesofunknownage. Pitman et al. • NESTING ECOLOGY OF LESSER PRAIRIE-CHICKENS 31 conducted throughout the range of the Lesser the latter was not a common occurrence dur- Prairie-Chicken. Giesen (1998) suggested that ing our study, but our methods did not allow nest success from those 10 studies was nega- us to locate nests that were depredated prior tively biased due to observer disturbance at to the onset of incubation. nest sites. Negative bias in our study was like- Few prairie grouse researchers have report- ly only slight because females were flushed ed nest success separately for first nest at- from their nests only once. Westemeier et al. tempts and subsequent renestings. Bergerud (1998a) reported that flushing incubating and Gratson (1988) hypothesized that preda- Greater Prairie-Chickens (T. cupido) once did tion of grouse nests was density-dependent not result in reduced nest success. Also, the and that renests would be more successful number of days between our initial nest visits than first nest attempts due to lower nest den- and predation events averaged >10 days. In sities. They also believed that nest success addition, only 2—% of the nests in our study should improve as new vegetative cover ap- were abandoned a much smaller percentage pears throughout the nesting season. Success than the 25% reported by Riley et al. (1992) of first and second nesting attempts of Lesser for Lesser Prairie-Chickens in New Mexico. Prairie-Chickens in Kansas, however, does not Further, one of five nests abandoned during support Bergerud and Gratson’s (1988) hy- our study was abandoned 9 days after the re- potheses, as first nest attempts were margin- searcher’s visit, indicating that it probably was ally more successful than renestings. Like- not due to human disturbance. wise, Greater Prairie-Chicken nests initiated in The percentage of females initiating a sec- Kansas prior to 30 April (presumably first at- ond nest during our study (31%) was between tempts) were more successful than nests ini- previous estimates forLesserPrairie-Chickens tiated after 1 May (presumably renests; Robel in New Mexico (15%) and Oklahoma (79%; 1970). Initial nesting attempts for Attwater’s Patten et al. 2005), and it was less than the Greater Prairie-Chicken (T c. attwateri) also 83% reported for Greater Prairie-Chickens were more successful than renests in 4 of 5 (Svedarsky 1988) and the 67% estimated for years (Lutz et al. 1994). Similar nest success Sharp-tailed Grouse (T. phasianellus\ Roers- for first attempts and subsequent renestings ma 2001). The percentage of Greater Sage- has been reported forGreaterPrairie-Chickens Grouse (Centrocercus urophasianus) initiat- in Colorado (Schroeder and Braun 1992) and ing a renest was highly variable (5 to 87%) Greater Sage-Grouse in Washington (Schroe- throughout their range (Schroederet al. 1999), der 1997) and Alberta, Canada (Aldridge and and most estimates were less than what we Brigham 2001). The only support for Berge- observed for Lesser Prairie-Chickens. Our rud and Gratson’s (1988) hypothesis comes models indicated that the low probability of from studies on Sharp-tailed Grouse in Min- Lesser Prairie-Chickens renesting in south- nesota and North Dakota, where success was western Kansas was influenced by the length higher for second attempts than first attempts of incubation before their clutches were dep- (Christenson 1970, Schiller 1973). In our redated (>50% ofunsuccessful initial clutches study, Lesser Prairie-Chicken nests initiated were incubated >12 days prior to predation). after 15 May were less successful (11.9%, n Similarly, Schroeder (1997) reported that = 42) than earlier nests (31.5%, n = 143), Greater Sage-Grouse in Washington whose regardless of nesting attempt. We speculate initial nests failed late in incubation were less that nests initiated after 15 May were less suc- likely to renest than those whose nests failed cessful due to an increase in predator efficien- earlier in incubation. Clutch size of the initial cy later in the nesting season, corresponding nesting attempt was also somewhat associated to changes in the structure and composition of with renesting probability in our study; how- vegetation. Cattle grazing began on our study ever, the magnitude ofthis effect was unclear. area around 15 May, and, after that date, grass The positive relationship that we observed cover and visual obstruction decreased sub- may have been due to increased fitness asso- stantially (JCP unpubl. data). Grazing coupled ciated with females laying larger clutches or with normal drought conditions during the the possibility that we misclassified some re- summer months in southwestern Kansas may nests as initial nest attempts. We speculate that result in declining habitat quality, and, there- 32 THE WILSON JOURNAL OF ORNITHOLOGY • Vol. 118, No. 1, March 2006 fore, the poor success ofrenesting LesserPrai- dance appeared to increase (estimated from rie-Chickens. Land management practices that casual roadside observations), as did nest pre- maintain taller and denser vegetation structure dation by snakes, in the later years (Pitman later into the nesting season may promote the 2003). Snakes may have been responsible for overall nesting success of Lesser Prairie- most partial-nest depredations because of the Chickens. lack of eggshell fragments at partly depredat- Clutch size in Kansas averaged 11.3 eggs ed nests. Also, three incubating LesserPrairie- — in 191 completed clutches greater than that Chickens were likely killed by snakes because reported in New Mexico (8.7) and Oklahoma their intact carcasses were found with a thin (10.8; Patten et al. 2005) or in 60 completed film of mucus covering the heads. In each clutches located in other states occupied by case, it appeared as if a snake had tried to Lesser Prairie-Chickens (10.4; Giesen 1998). swallow the bird. Our study is the firstto document substantially Interspecific nest parasitism has been re- different mean clutch sizes for first nests (12.0 ported for Greater Prairie-Chickens and eggs) and renests (7.6 eggs). Merchant (1982) Sharp-tailed Grouse (Leach 1994, Westemeier reported mean clutch size for initial and sec- et al. 1998b), but had not been reported for ond nesting attempts, but his estimates were Lesser Prairie-Chickens before our work in similar for both (9.8 and 10.7 eggs, respec- Kansas (Hagen et al. 2002). Only 6 of 209 tively). In our study, the percentage of eggs (3%) nests were parasitized by Ring-necked containing a developed embryo was 94% and Pheasants and/or Northern Bobwhites, and 2 hatching success was 74%. Egg fertility has of the 6 (33%) nests produced Lesser Prairie- not been reported previously for the Lesser Chicken chicks. Hatching success of eggs in Prairie-Chicken, but hatching success of eggs these two nests was 72%, similar to the 74% was estimated at >90% across three studies estimated for 46 unparasitized nests (Hagen et (see Giesen 1998). The lower hatching suc- al. 2002). Our study provided no evidence that cess observed in our study reflects partial nest nest parasitism negatively affected nest suc- losses that occurred in 32 of 48 (67%) suc- cess or hatchability of Lesser Prairie-Chick- cessful nests. ens. Identifying nest predators from nest re- Bergerud and Gratson (1988) hypothesized mains is difficult because patterns of egg that successful female grouse would nest in breakage overlap among, and even within, the same area in the subsequent breeding sea- predator species (Lariviere 1999). Uncertain- son. In southwestern Kansas, female Lesser m ties were reduced on our study area, however, Prairie-Chickens nested within 712 of the because coyotes and gopher snakes were the site of their previous year’s nest site (if suc- only common species capable of preying on cessful). This degree of philopatry is similar Lesser Prairie-Chicken nests. Studies in New to that reported for Greater Sage-Grouse in Mexico and Texas revealed that Chihuahuan Wyoming (Berry and Eng 1985) and Idaho Ravens (Corvus cryptoleucus), badgers (Tax- (Fischer et al. 1993). Greater Sage-Grouse in idea taxus), striped skunks (Mephitis mephi- Washington showed less philopatry to a pre- tis), and ground squirrels were the primary vious year’s successful nest location, moving m predators ofLesser Prairie-Chicken nests (Da- an average of 1,600 in the subsequent nest- vis et al. 1979, Haukos and Broda 1989, Riley ing season (Schroeder and Robb 2003). et al. 1992). However, few corvids, badgers, The association between lek location and or striped skunks were observed on our study nest placement has important managementim- area, and, although ground squirrels were plications for identifying critical nesting hab- abundant (estimated from casual roadside ob- itat. Bradbury (1981) hypothesized that fe- servations), they were identified as important male home ranges included only one lek and nest predators during only 1 year (2000). that >50% of all females should locate their Davis et al. (1979) documented snakes nests nearer to that lek than other nearby leks. preying on Lesser Prairie-Chicken nests in Studies of Greater Sage-Grouse and Sharp- New Mexico. We found little evidence for tailed Grouse have provided support for this snake predation ofnests during the early years hypothesis (Bradbury et al. 1989, Giesen of our study (Jamison 2000), but snake abun- 1997). In Colorado and Minnesota, however.
