Molecular phylogeny and color-pattern evolution of Vanessa butterflies (Lepidoptera, Nymphalidae) PDF
Preview Molecular phylogeny and color-pattern evolution of Vanessa butterflies (Lepidoptera, Nymphalidae)
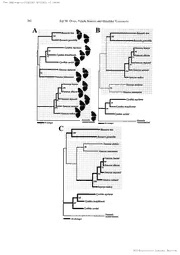
TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan uttlk Trans.tepid.Soc.,Iopan57(4):359-370, Septernbcr2006 Molecular phylogeny and color-pattern evolution of Vhnessa butterfiies (LepidopteNrymap,halidae) JQji M. OTAKi')・2) Y*U,ichi KiMuRA2' and Haruhiko YAMAMoTo2) '' Departmen tof Chemistrl Byi,olegy, and Marine Science ,Faculty of Seience, Universi toyf the Ry ukyus, Nishihara ,Okinawa, 903-02 13 Japan !) Departrnent of Biologica lScicnces ,Kanagawa University, 2946 Tsuchiya,Hiratsuka,Kanagawa, 259-1293Japan Abstract Experlmental treatment of pupae with cold shock or tungstate, a protein-tyro spih]ose- inhibitor, in butterflies. phatase produces a series of unique wing color-pattern modifications adu]t Being similar to the tungstate-induce dmodifications, several Vanessa species can be arranged in a series of the systematic color-pattern dilferen c1easrge lbyased on the re]ative area of orange (RAO) on the forewings .The hypothettca lrnolecular pathway that is sensitive to cold shock or tungstate has been proposed to be involved in speciation of thes ebutterfi iHeesr.e we investiga tpehdylogeny of llaness aand its related butterfli eisn reference to DNA sequences of thc mitochondrial AL`IDH deh.vdrogenas esubunit 5 gene and c),tochrome oxidase subunit J gene ,Seven species tha tbelon gte the conventional Vtiness gaenus were separated int otwo groups: Five species, V. indica ,V, h'amani, V. dqieanii ,V. dileeta ,and V/ buana. formed an indcpendcnt cluster with strong bootstr aspupport ("th iendic agroup"), excluding V. atalanta and V. tameamea as a separate clade ("th aetalanta group"). Thus, the indica group contains five species with various RAO values includin Vg, scrmani (whic hhas larg eRAO value) and V. dojean i(iwhic hhas small RAO value). Sirnilar tlhye ,atalanta group consists oftwo species, V. atatanta and V, tameamea, whose RAO values are quit edifferent l'rom each other, Our data argue for the evolutionary model in which the conventional Vkenessa genus, but not it srclated genera, has a unique tendency to evo]ve "bi-directionally" to species with large or small orange area on thc forc",in gbesyend phylogenetic constraint from either the indica or atalanta groups within the genus. Key words Lepidopter aN,ymphalidae ,i/?ines smoal,ecular phy]egeny, color-pattern evolution, relative area of orange, speciation, cold shock. Introduction The spectacular variety of butteri lwiyng color-pattems nicely illustrat etshe geneti cpoten- tia lof organisms for evolving int othousands of species with divers ephenotypes ,The wing color-patterns of many butterf ispyecies functio nas visual signals for mates or predators, and accordingly, they are importan ttrait sfbr natural selection (Uesug 1i9,91; Jiggins et at,, 2001; Kapan, 2001) .Only their rough pattern sb,ut not detaile pdatterns ,had been consid- ered to be functiona lat least in some species of nymphalid and papilioni dbutterflies (Tinber gete ang., 1942; Hidaka & Yamashita ,1975) ,but recent studies reported that more delicat edifference scan be discriminated in q lycaeni dbutterf i(yFordy cete at,, 2002), Studie son butterf iwiyng color-pattems and speciation will give us an insigh itnto the dy- namic evolutionary histor ywoven by natural selection and other factors, From the viewpoint of developmenta ]biology t,he mechanism of the eyespot formatio non butterfl ywings has been investigate dextensively with surgical, physiologic aaln,d molecu- larbiologica]techniques (Nijhou1t9,84, 1991; French & Brakefietd,1992, 1995; 'Corresponding ± author: [email protected] NNIII-IE-leEcltreoncitcronic LMbirabrryary Service TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan 360 Jeji M. OTAKi, Yuichi Klh・ft/ RaAnd Haruhiko YAMAMoT'o Brakefiel&d French,1995;Brakefield 1996). There datain- et al,, are much experimental dicatin tghat an eyespot focus on the wings works as an organizing center for the color-pat- tern development ,although the exact nature of the position aslignal emerging from it is largely enigmatic. The same organizing center seems to organize the pupal wing cuticle spot (Ota keit al,, 2005a). In addition, color pattern smay be determine dnot only by the putativ peesitiona lsignal fror norganizing centers but also by the cellular ability to interpret the position aslignal, which could be modified by more systemic factor ssuch as ecdys- teroids (Koc h& BUckmann, 1987; Koch et al., 1996; Rountree & Nljhout, 1995) and the putativ ceold-shock hormone (Otak 1i9,98, 2003; Otalc &i Yamamoto, 2004a), We have been interest eidn the mechanism of wing-wide color-pattern determinati odnuring development and it srelationship with butterf levyolution. In this context, phylogenetic rela- tionshi pasmong the genus llaness Faabriciu s1,807 in the narrowest sense (Itlane ssenssatt stricto) are of particul airnteres tb,ecause a systematic co]or-pattern modification tendency is obse]'ved in this genus ,More precisely ,one can arrange several species of linness ab,ut not the species of it sclosely-related genera Civnthi aFabricius ,1807 and Bassaris Hifbner, 1821, as a progressive series of the color-pattern difference spartl ybased on the quantitative value of "the relative area of orange (RAO) "on th edorsa florewing s(Ota l&( iYamamoto, 2004b) .The serial order of species from the widest orange area to the narrowest area with the RAO value (±SD) as an indi-cat oirs :liZines ssaamani (Hagen ,1895) (23.±71.092]), lilrnes staameamea Eschscholt z,1821 (20.±92,5%), iikenes saindica (Herb st1,794) (19,81.3%),iilanessdailectaHanafusa,1992(16.01.19Z),Vlrnessa (Linnaeus, ± ± atatanta 1758) (13,±8O.6%), and VZiness daojeani iGodart ,1824 (10.±6O.9%) (Otak i& Yamamoto, 2004b) .Based on this fact t,hey can be ciassified into three co]or-pattem types: orange type (V .samani and V. tameamea, with larg eRAO values), intermedia tteype (V .indic aarid V, dilect aw,ith intermedia tReAO values), and black type (V .atatcuzta and V. dojeani iw,ith small RAO values) (se eFig. 2). This color-pattern series (Ota k&i Yamalnoto ,2004b) does not seem to refiect moiphological phylogeneti cresults derive dfrom the examjnation of genitali(aFie]1d9,71;Leestmans, 1978). Intriguing lay s,imilar color-pattern series is known to be induced by systemic iajectio nofs sodium tungstate ,a protein-tyros ipnheosphatas e(PTPase i)nhibito irn,to pupae of V, indica (Ota k&i Yamamote, 2004a), This modificatien-inducing ability of tungstate is shown to be large] yspecific to the color-pattern development ,and such modifications are not observed in the iajection sof stress-inducing chemicals such as urea (Otak i1,998), thapsigargin ,ieno- mycin, and geldanamyci n(Ota keti al., 2005b) ,Thus, a signal transductio npathway fbr the color-pattern determination in which the tungstate-sensiti vPeTPase plays an instructjv reole has been proposed to be involve di'n speciation of lhnessa butterfii e(sOtak &i Yatnamoto, 2004a, b;Otaki 2005b), et al., Here we phylogenetical elxyamined buttertl iofe sthe genus Vlaness saenstt stricto and it sre- late dgenera CyJnt hainda Bassari sin reference to DNA sequences of the mitochondrial AL`IDH dehydrogenase s"bunit 5 (ND5 )gene and cytochrom eoxidase subunit I (COD gene which are known to be reliable as a "motecular clock" with high variability even between closely-related species (Sperli n2g00,3). AIthough the transpecifi ccolor-pattern differences in Vkeness aor any other genera are not likel yto refiect simple orthogenesis of the genus (Shapir ol,984) ,we especially fbcused on relationships, if any, between the color-pattern dillerenc eansd molecular phylogeny .Possibl eevolutionary history of S/lanes sbautterfii eiss discusse bdased on the molecular phylogenetic results together with morphological, ecolog- ical a,nd biogeographic aplerspectives. NII-Electronic Library Service TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society oofJfap anJapan 361 VZinessa Phylogeny and Color Pattern Material sand methods Classification and specimens Standar dliterat uwraes referred to for phylogeneti scystematics of butterfl i(eHsarve 1y9,91 ; de Jong et al,, 1996; Ackery et al., 1998). Three terms for morphological genera ,lr2znessa, C},nth ainda ,Bassari s(Fiel 1d9,71) ,were used fbr the sake of discussion ,althoug hrecent molecular phylogeneti dcata argue that these genera are to be treated as a single genus vanessa (Wahlbe ertg al., 2005). There are fiv especies in the genus liZines tshaat were recognized in Field (1971 )V:, indica, V. atatanta, V. tameamea, V. dojeani ian,d V, samani. According to Field (1971 )V,, indica has fiv esubspecies, two of which are V. indic abefan a(Fruhsto r1f8e98r) ,and V. indic avul- cania Godart ,1819 (Fiel d1,971) .However, Leestmans (1978 p)roposed that V. buana and V. vulcani.a are indcpenden tspecies. Recently ,a new species, V dilect aw,as described (Hanafu s1a99,2) .Moreover, Antanarti aab.yssinica (Felde &r Fclder ,1867) has now been include din the lttines sgaroup as Y. abyssinica (Wahlbe ertg al,, 2005), In total, there are nine species of l,Zines stahat have been recognized thus far. We obtained 13 specimens of butterf lfireosm Prot insect s(Osak aJ,apan) ,The Insect Company (Woodfal lUs., K.) ,or our own collection includin gseven VZi.nes saspecies (in- cluding V. buana but excluding V. vulcania and V, ahyssinica), two Bassaris species (B, ite a(Fabric i1u77s5,) and B. gonerilta (Fabrici u17s7,5)) ,and three representative qr・nthia species (C ,caTdui (Linnaeu s1,758) ,C. hralilien si(sMoor e,1883) ,and C, myrinna (Doubleda y18,49)) together wjth ntnonia westermanni Westwood, 1870 ([Ib b1l)e. Molecularbiologicatlechniques [[bt aDlNA was extracted from drie dspecimens of butterfii eussing DNeasy Tissue Kit (QI- AGEN). Extracte dtota lDNA was subjected to polymeras echain reactions (PCR )under the followin gcycling conditions using Rfr DtNA polymeras e(Promega )f:or ND5, initi adlena- turing of 94eC for 3 minutes, 30 cycles of 940C for 1 minute, 46eC for 1 minutes, and 700C for 2 minutes, and the supplementary extension step of 700C for 12 minutes; for COL initial denaturin gof 950C fbr 5 minutes, 40 cycles of 940C fbr 30 seconds, 470C fbr 30 seconds, and 720C for 1.5 minutes ,and the supplementary extension step of 72CC fOr 10 minutes, PCR primer sfbr ND5 were designe dbased the ND5 sequence from Vlaness iandic acaught Table1. Adult buTterfl yspecimens used in this study. Species SpecimenID Locality Date caught acccssion no, in the fieldGenNBDa5nk COJ V.indicaV. JMOOOOI (KU)Kanagawa,Japan Jun.2004 DQO28749 DQ385858 b"anaV. JMOOO02 (KU) Sulawesi,Indonesia Aug.2002 DQ0287SO DQ385867 dojeanii JMOOO03 (KU) Mt Lawu, CentraJlava,IndonesiaOct,2000 DQ028751 DQ385862 V. tameamea JMOOO04 (KU) Kttuai ,Hawaii May2000 DQ028752 DQ385861 V. samani JMOOO05 (KU) Sumatra.Indonesia unknown DQ0287S3 DQ385863 V. dilecta JMOOO06 CKU) Mt Mutis, Timor unknewn DQ0287S4 DQ385864 V, atalanta .rMoooo7 (Ku) Siox,akiaMelbourne, Aug.2000 DQ0287S5 DQ385860 B,iteaB. JMOOO08 (KU) Australia Feb.1996 DQ028756 DQ385859 goneriUa JMOOO09 (KU) Auckland ,New Zealand Feb.2003 DQ028757 DQ385g65 C, cardui JMOOOIO(KU) Kanagawa, Japan Dec.2004 DQ028758 DQ385856 C. myrinna JMOOOI1 (KU)Huallega P,eru Mar,20el DQ028759 DQ385857 C. braziliensiJMsOOO12(KU) Tingo mana, Peru jane2003 DQ028760 DQ3g5g66 J, westermanni JMOOOI3(KU) Bangui. Centra Alfrica Julyl981 DQ028761 DQ38S855 NII-Electronic Library Service TThhee LLeepipdiopdteorpoltoegircaollSoocgleitycal Society oofJfap anJapan 362 Joji M. OTAKr, Yuichi KiMuRA and Haruhiko YAMAMoTo -lochsnges lvestermannt -toch#"ges westerm"nm - 10 changes NII-Electronic Library Service TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan Vtinessa Phylogeny and Color Pattern 363 in Shimane, Japan ,that had alread ybeen known (GenBan Akccession Number AB175865) as fbllows :5'-GTTCMTCAIiCTACTT[[AGrlAACTGCTG-3' and 5'-AAGGAMACCA- CtOffAAAGC[[AAArTTG-3'. A part of the ND5 gene (35 7bp excluding primer sequences) was amplified by PCR. DNA sequence of the PCR produc tfrom Vanessa indic acaught in Kanagawa showed the perfec tmatch with the sequence of the same species already in GenBank (AB175865 s)ug,gesting that variations within thi sspecies in Japan may be negli- gible .For COI, widely used PCR primers ,5'-GGTCAACAAMCAI]AAAGATATTGG-3' and 5'-TAAACTTCAGGGTGACCAAAAAMCA-3', were employed, ampljfying a 667- bp stretch (exclud PiCnRg primers) of COJ. Although we cannot exclude the possibilit tyhat the ND5 and COI gene sequences vary within a given species, we here assumed that such variations are not larg eenough to override variatjons between species, This is partl ybe- cause many ltlanes sspeacies are fbund in restricted areas with relatively small and hence ge- netically homogeneous population san,d also because it is diMcult to obtain multiple speci- mens of these rare species from diifere nretgjons. PCR product swere cloned into pCR-BIunt II-TOPO (Invito goern p)CR4Blunt-TOPO (Invitro ganedn )sequenced using M13R and M13F primers in Dragon Genomics Center (TAKARA BIO, Yokkaichi ,Japan) .These sequences were deposite din GenBank, whose GenBank Accessio nNumbers are shown in [fabl e1. Phylogeneti canalysis Validit yof the use of the ND5 and COI genes in studying phylogeny of insect hsas already been demonstrated (Sperli n2g0,03) .For the sequence analysis, we used a 357-bp stretch of the ND5 gene plus a 667-bp stretch of the COI gene (tota 1l0:24 bp) excluding PCR primer sequences. In making multiple alignment, ClustalXl.8 3(Higgi n&s Sharp ,1988; Tho- mpson et al., 1997) was employed, Phylogenetic trees were drawn using RilU]P* 4.0ie 10 CSwoffor 2d0,00) .We here compared three tree diagrams that were derive dfrom djfferen tclustering methods, from a meta-ana- lytic point of view. For constructing the neighborjoining (NJ )tree diagrams ,Kimura's two paramete rmethod (Kimura ,1980) was used for calculating distances .Rate fbr variable sites was set as "equal". For constructing the maximum parsimony (MP) tree diagrams ,the fu1 1heurist itcype search method was carried out with the TBR (tr ebeisectio annd recon- nection) algorithm, The maximum likeliho o(dML) tree diagrams were also constructed using the same software with the GTR model. The bootstra cponfidence leve lofbranching in trees was obtained with 1OOO resampling. Results and discussion Relationship asmong Vkenessa ,(lynthia ,and Bassaris Our primary purpose in thi spaper is to delinea teevolutionary histor yamong species in the Fig, ], NJ (A) ,ML (B) ,and MP (C :most parsimoniou str)ee diagrams of Vdnessa and it srelated gencra with Junonia westermanni as an outgroup. Thick lines indicate dclades with at least 50% bootstrap support (A-C) ,The bootstrap values, shown in these diagrams, were calcu- lated bascd on 1000 resampling. Two groups can be recognized: the indic agroup and the atalanta group. Bassaris ,C.vnthi aan,d the indica group formed stable clusters in all dia- grams, whereas the ataianta group was unstable. Relationship samong species within each clade were stable in all diagrams, but relationships among four groups (i .e, Bassaris, Cynthi tah,e indica group ,and the ataianta group) were ditferen itn three mcthods. NII-Electronic Library Service TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan 364 JojiM. OTAKi,Yuichi Km{ukx HarnhikoY,m{.・"foTo and I,kenes gseanus and to fin dout relationships between the color-pattern differenc eansd phy- logeny ,However, before discussin tghat topic ,it is reasonable to examine phylogeneti rce- lationshi pamsong three genera ittines sday,nthi aand, Bassaris b,ecause whether or not the independenc eof these genera is phylogenetica lvlalyid is somewhat controversial. On the other hand, we here assumed the monophyletic status of the entire i,lrnes sgaroup (ilanessa+dynthia+Bas ass asrhoiwns) in Wahlberg et al. (2005). After making the multiple alignment of the DNA sequences from butterfl ioefs Vkenessa, C>Jnthi aBa,ssaris, and Jinnoni (al 3species shown in Table 1 and Fig. 1) ,we found no vari- ation in lengt hthroughout species. We then constructed molecular phylogeneti tcree dia- grams using a sequence from j. westermanni, which belongs to the tribe Junoniin it,he sub- family Nymphalinae (WahIbe regt at,, 2005) ,as an outgroup (Fig .1) .The use of J. i・vester- manni as an outgroup can be justif biye tdhe fact that all other samples belong to the tribe Nymphalini, which is a sister group of Junoniin (iWahlber egt' al., 2005), In alr diagrams derive dfrom differe nmetthods, the cluster of five species (V ,indica ,V. samani, V. dojeani iV,, diiecta ,and V. b"ana) was well supported by the bootstra pvalue, 100%. We call these fiv especies in this clade "the indica group" .Tsvo other species, V, atalanta and V. tameamea, were excluded from the indic agroup, and we called these two species "the atalanta group", althoug htheir monophyletic status was Qnly weakly support- ed. Noting that the examination of genital iiandicated the closeness of these two species (Leestman s19,78), it is not unreasonable to consider them as a distin cgtroup, because tbey were nonetheless excluded from other I,kenes sspaecies and closely located in al 1phyloge- netic tree diagrams that were examined. Hence, these seven species that belong to the con- ventional }irnessa genus were divided into two groups and did not fbrm a monophyletic group in any diagrams .Three (;>,nth isapecies fbrmed a c]uster with reasonably high boot- strap support in all three methods. Similarl ytw,o Bassaris species formed a distinc tcluster with high bootstrap support, 100%, in all thre ediagrams. However, c]ear relationships among these four groups (i e.. Ciynthi aB,assaris, the indic agroup and the atalanta group) were not specified in any methods. [[bgether o,ur data clearly indicated the dithcul tiyn specifying relationships among the gen- era, vanessa ,Cynthi aan,d Bassaris. This conclusion may be compared with a recent mo- lecula prhylogeneti acnalysis on nymphalid butterfli eisncluding two lhnessa species (V ,in- dica and V. atatanta), fiv eq,nth ispaecies, and two Bassaris species, in which the classical lhnessa ,Bassaris, and most (:lynth isapecies except Cynthia annabella Field ,1971 each formed independen tclusters (Wahlbe ertg al,, 2005). However, Iargely because of the cryp- tic and unexpected phylogenetic status of C. annabeUa and A. abyssinica, three genera, I,kenes s(a[,lynthi aa,nd Bassaris were proposed to be grouped as the single genus Slanessa (Wahlbe ertg al., 2005) .It is also noteworthy that V. indica and V. atatanta were shown to be sister to each other in Wahlberg et al, (2005 )T.hus, our phylogenetic diagram that was derive dfrom the MP method (Fi gI.C) is most consistent with Wahlberg et aL (2005). However, Wahlberg et al, (2005 u)sed only two species from the genus Sittnes ssaenstt stric- to. The relationships in thi sgenus ,the most importan ttopic in this paper ,arc discussed below. Speciesrelationships inthegenuslhnessa Although our phylogeneti tcree diagrams obtained above were not able to clarify relation- ships among the morphological genera ,they provided us with important informatio tno ex- amine species relationships in the genus Itlanessa. As discusse dalready, fiv el,lrnes sspaecies CV .indica V,. samani, V. dojeani iV,. dilect aa,nd NII-Electronic Library Service TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan Vtinessa Phylogeny and Color Pattern 365 V. buana) formed an independent cluster, the indic agroup ,with high bootstra pgupport 100% in all clustering methods <Fig ,1) ,indicatin gtheir ditferen tevolutionary path from that of the atalanta greup ,V. atalanta and V, tameamea, In the indica group, the fbur species were shown to be a sister group in relation to V, indic awith reasonably high boot- strap support in all three methods. Among them, it was somewhat surprising to find that V, indic aand V. buana were not shown as the closest relatives to each other despit ethe fac tthat they had been considered as the same species until Leestmans (1978 ).Thus, the current morphological systematics (Leestman s1,978) is indeed consistent with our molecular result. On the other hand, V, dilect aand V. b"ana were sho"rn to be very close to each other in all three methods. Their color-patterns are also very similar (Fiel d1,971; Hanafusa, 1992), It may be possibl eto consider them as two subspecies ef V. buana: V. buana bttana and V. buana dilect afrom the viewpoint of taxonomy, AIternatively i,t is theoreticall pyossibl eto treat the whole indi- ca group as a "superspecies" from the viewpoint of evolutionary biology, It is worth while to point out that V. samani, V. dojeani ian,d V. b"ana fbrm a species group differe nftrom V. indic aand V. vtttcania, based on the morphological examination of geni- tali a(Leestma n1s97,8) .Since within the indica group, a group of V. samani, V. dqieanii, and V, buana together with V. dilect ias sister to V. indica o,ur phylogeneti rcesult is consis- tent with the conventional systematics. Molecular phylogeny and color-pattern evolution Visual combination of the color-pattern analysis (o ranalysis of the phenotype-base tdraits) with the molecular phylogeneti acnalysis (o ranalysis of the genotype-base d"traits") helps us to understand species relationships clearly (Fi g2.). The indica group contained species with larg eRAO value (i ,e. V. samani, belongin gto the orange type), one with small RAO value (i e,, Y, dojeani ib,elonging to the black type) ,and ones with intermedia tReAO val- ues (i e.. V. indica ,V dilect aan,d V, buana, belongin gto the intermedia ttyepe). Likewise, the atatanta group contained two species with quit edissimil acrolor-patterns: one with 1arge RAO value (i e.. V. tameamea, belonging to the orange type), and the other with smal1 RAO value (i e.. V. atalanta, belongin gto the black type). This immediatel yindicate sthat the color-pattern series based on the RAO values does not simply refiect the molecular phy- logeneti rce]ationships. Since butterf lcoylor-patterns can be drastical dliyffere nbtetween closely related species or even within a single species, they are not considered as useful traits for clarifying phylogenetic relationships. Hence, it is not surprising to find no simple correlation between the color-pattern di{ferenc eansd the molecular phylogenet ircesults. Nonetheless, Fig. 2 readily indicat etshat even within a group of closely related relatives, the color-pattern can drastical vlayry, but it svariation is always on the lin eof the simple color-pattern series. In order to recapitulate the plausib leevolutionary histor yof these butterfii wees, here pre- sume the existence of the imaginary prototyp eof 1laness ian the past ,which was morpho- logical laynd geneticall ysimilar to V. indica .This presumption w,hich does not contradict our molecular phy]ogenet ircesults, was made based on the followin greasons. First ,V, in- dica is wide]y distribute din the Orienta land Palaearcti cRegions ,and it has the largest number of subspecies (fiv reecognized subspecies including V. indica huana and V. indica vutcania, according to Field (1971 i)n) the genus Linnessa .This fac tindicat etshe versatility o'f V indic ato adaptitselfto differen tenvironmental conditions. Second, V. indic aoccupies difTer- the middle positio nin the ]inear progressi vseeries of the quantitati cvoleor-pattern ences (Ota kaind Yamamoto, 2004b) ,again meaning that it may not be a species which is very differentiat etdo particula renvironmental conditions and hence it may still retain the NII-Electronic Library Service TThheLee piLdeoppteirdoloopgitcaelrSooclieotygical Society ooff JJaapapnan 366 JQj iM. OTAKi,Yuich iKiM(JR Aand Haruhiko YAMAMoTo ATALANTA CROUP N e Usatnani Mtameamea X inttieaeZ dilecta Uatatanta Udojeanii ORANGE TYPE SNre"ERrVifEbgtN'F・}-e >ri e.Ps EXEb)tianBaLACK TYPE INDICA GROUP Fig,2. Visual combination of the color-pattern seTics and molecular phylogeny of the Vdnessa species. Phylogenetic rclationships are indicat ebdy lines and an ai'row. Bascd on the color- patter dniiferenc esesvc,n Vliness aspecies can be divided into three types :orange type, in- termediate type, and black type. Both indica and atatanta greup scontained species of dif feren tcolor-pattern types. }'vEsibk).,i・lige'knlIE-lnevl-IL・ ig P i r ri a e b Au .ffni.at,.1 g ; ・\Ig'ag}:tet':} ' e sulawesl " ,t S・fggs'?,se'Fdsgsfgfiftlyei+jaSr3ya'g.v, ft.) Bmli Su4}llmxn. ' 'ila:.s/"'//- g'・' x' . Lombok t.t P'Ztne sdsoajeani t l 'g;・s,,i.f .s,itsiLf',.','・ci..,}t''t;,,.k・'s'' Fig. 3. Segregate ddistrjbutio nosf four Vkenessa species in the Indonesian Islands ,V. samani is found in Sumatra, V, dojean iiin Jawa, Bali, Lombok, and Sumbawa. V. dilect ain Timor, and V, buana in Sulawes i.However, their distributi aornes all restricted to smail mountain- ous regions within a given island .Simi]arl yV,. tameamea is confined to mountainous re- gions of the Hawaiian Islands (no tshown). Butterfi ynames and their corresponding distri- butio nareas are color-coded. Coincidentally ,geographic adlistance sfrom the Asian Continent to each jsland that harbor a given species secm to be correlatecl with molecu]ar phy]egenet diicstances from the root of the indica group. NII-Electronic Library Service TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan Vtiness Pahylogeny and Color Pattern 367 potenti aflor futur especiation. This imaginar yancestor similar to Vl indica might have evolved int oseyeral species with a larg espectrum of color-pattern differenc eosn:e to a larg eRAO value and the other to a small value. This color-pattern evolution might have been shaped by differe nentvironmen- tal factor fsor each species. And one of these factor msay be temperature fluctuatio tnhsat produce natural cold-shock events. This speculation is based on the fac ttha tthe tungstate treatment ,which is equivalent to the cold-shock treatment ,of V. indic acan produce a series of phenocopies that are similar to other lhnessa species, It is importan tto recognize that V. tameamea, V. samani, V, dojeani iV,. diZect aa,nd V, buana are all fbund in mountainous re- gions of tropica lisland susually above around 4,OOO fee t(1,20 0meters) (Fiel d1,971). [femperature fiuctuati ownhi,ch is higher in high altitudes than in low altitudes and also higher in low latitude sthan in high latitud esma,y act as cold-shock events to drive a preces sof evolution. However, the environment that produces natural cold-shock events to pupae may be necessary but never be suMcient for the wing color-pattern evolution of Vkeness a.[[b be sure, it may be necessary for us to differenti tahtee cold-shock conditions and simpie low temperature conditiong in discussing the color-pattern modifications and (Otaki 2005b). evolution et al,, It is importan tto realize that the expansion or shrinkage of the orange area on the forewings during speciation occurred independentl ayt leas tfbur tjmes (V .atalanta, V. tameamea, V. samani, and V. dojeanii i)n this genus ,With the genus ,this color-pattern evolution hap- pened irrespecti ovfe the phylogeneti sctatus, Furthermore U, dilect aand V. buana may be on the verge of such differentiati onT.hat is ,in the course of speciation, as long as it re- mains a species of l,laness aan, emerging new species could freel yenlarge or shrink the or- ange area at any phylogeneti sctatus, But no other way of co]or-pattern evolution seems to be allowed. This L`bi-directional" type of color-pattern evolution cannot be found in Cynth iora Bassaris, and it appears to be unique to this genus, despit ethe fac tthat other nymphalid butterfl iaeJsso show similar color-pattern response to experimental treatments (Nijhou 1t9,84; Shapiro ,1984; Otaki ,1998) .Howeyer, somewhat similar evolutionary his- tory may be found in Mac"tinea butterfli wehsic,h belong to the family Lycaenida e(Otaki and YtLmamoto ,2003) ,The origin of the various color-pattems in the genus Sxlanes msaay be explained, for example, by the differen texpression level sof the putativ ceold-shock sen- sitive facto rthat plays a role in the color-pattern determinati oorn development. This bi-directio ntayple of color-pattern evolution that was observed only within Lirnessa sensu stricto but beyond the constraint of the phylogenet igcroups (i. eth.e indica and ata- lant agroups )may be explained by a two-step process: the restriction of developmental plastici otfy Litnessa sensu stricto followe dby opportunistic changes ef expression level sof a gene responsible for the RAO value. The latte srtep, which may be considered as a kind of moiphologica] convergence, appears to have some similarities, at leas tat the conceptual level t,o the convergent evolution of the black spots en Drosqphila wings, In the case of the Dtvsophil awing spots, activation or inactivatio nof the pleiotrop ipcigmentatio ngene yet- tow on the wings that was controlled by the cis-regulatory element is responsible fbr the convergent evolution (Prud'hom mete al,, 2006) .A similar melecular mechanism might be expected in the case of the color-pattern evolution of Iiinnessa, Biogeographical perspectives Biogeographical distribution osf I,lanes ssapecies are quite unique. There are two species that are distribute djn a fairl ylarge geographic aalrea: V. atatanta and V. indica ,The for- mer is distribute dwidely in Europe and North America, and the latte irn Asia and other sur- rounding areas. In contrast, V. tameamea is fbund only in the Hawaiian Island sa,nd in the NII-Electronic Library Service TThhee LLeepipdiopdteorpoltoegircaollSoocgieitycal Society ooff JJaapapnan 36g JQji M, OTAKi, Yuichi KiMuRA and Haruhiko YAMAMoTo island si,t prefer smountainous areas (Fiel 1d9,71; Jamjeson and Denny, 2001). The other species, V, samani, V. dojeani iV,. dilect aa,nd V. huana, are all found in the Indonesian Island bsut in differe nitsland wsith no overlapping distributio anreas. Biogeographic adlistributi oofns the four species tha tformed the indica group in our phylo- geneti ctree diagrams, V. samani, V. dojeani iV, dilec' tanad, V. b"ana are shown in Fig ,3, They are parapatt iicn thi sbiogeographical lcoymplex region (Vatne-Wr i&g hdte Jong, 2003) :V. samani i' sfound in Sumatra Island ,V, dcieani iin Jawa (Java B)a,li ,Lombok, and Sumbawa, V, buana in Sulawesi ,and V. dilect ian Timor. Their morphelogical relatives in the Australia Rnegion ,i ,e, two Bassaris species, are not distribut iend these island si,ndi- cating the differe nhtistor yof establishing current distributions, The imaginar yancestor of the genus Vtiness adiscussed above probabl ymigrated from the Orienta lRegion to Indonesi a,Subsequent world-wide climate change probably caused physica lisolati oofn their imaginar yancestor in high altitude regions of island sthat occa- sionally produce natural cold-shock events. Coincidental ]tyhe, molecular phylogeneti rce- lationshi p(so rphylogenet idcistanc efrom the root of the indic agroup) seem to recapituJate biogeographical distributi o(nos rgeographical distanc efrom the Asian Continent o)f the in- dica group ,That is ,the pair of V. b"ana and V. ditect ap,robabl ythe most recently di- verged species within the indic agroup, is fOund in islands very remote from the Asian Continent ,V deieani ip,robabl ythe second most recently diverge dspecies, is found in is- lands closer to the Continent .V. samaniis fbund in an islan dwhich is even closer. This historic aanld biogeographic arlecapitulation is consistent with the speculation that the an- cestral species migrated from the Asian Continen tand that the co]d-shock-sensitive factor played a role in the wing color-pattern evolution of these Vkeness aspecies. Acknowledgments We thank Kojiro Shiraiwa ,who rhaintains a beautif uw1eb site, "Pteron Wbr!d", fbr kindly providing us with specimens. We also thank Hideomi YUi, directo orf "STAGE", for various drljls:ccuhsnoslioogny s.This work was partly supported by Kanagawa Academy of Scienc eand Research Grant ,by lndustry-Academi cCollaborati oPnreject of Research Institut efor Integrate dScience, Kanagawa University ,by the Environmenta lResearch Grant from The Sumitomo Foundation ,and a]so by the 21st century COE program of the University of the Ryukyus from the Ministry of Education, Culture ,Sports ,Scienc eand Technology,Japan. References Ackcry. P., de Jong, R, and R. I. Vane-Wright, 1998 . The Butterflie sH:edyloidea, Hcsperioidea and Papilionoidea .in Kristense Nn.. P. (Ed, )Lc,pidoptera ,moths and butterfi i1e. s.Evolution, systematics and biogeography. Handb, Zool, Berl, 4 (35 )2:63-300, de Gruyter B,erlin. Brakefield ,P. M. and V. French .1995 .Eyespo tdevelopment on butterfl ywings: the epidermal response to damage. DeL,l Biol. 168: 98-11 L. Brakefield ,P. M., Gates ,j. ,Keys, D., Kesbeke, F., Wijngaarden, P. J. ,Monteire, A., French, V. and S. B. Carro]) ,1996. Development ,plastieit aynd evolution of butterf elyyespot patterns ,Nk;t"re 384: 236- 242. de Jong, R., Vane-Wright, R. I .and P. R. Ackery, 1996. The highe rc]assification of butterfl i(eLsepidoptera): problems andprospects. Entomologica seand. 27: 65-101. Field ,W. D., 1971 .Butterfl ioefs the genus Vtmessa and of the resurrected gener aBassaris 'and C>'nthia Cepidopter aN:ymphalidae), Smithson .Contr .Zbot. 84: 1-105. Fordyce ,J .A., Nice ,C. C, ,Foristc Mr,, L, and A. M. Shapiro, 2002. The sjgnificance of wing patter dniversi- ty in the Lycaenidae: mate discriminatio nby two recent]y diverged species. J. evol. BioL 15: 871-879. NII-Electronic Library Service
