// /l'/Z PDF
Preview // /l'/Z
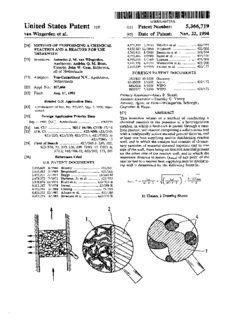
US005366719A United States Patent [19] 5,366,719 [11] Patent Number: van Wingerden et a1. [45] Date of Patent: Nov. 22, 1994 [54] METHOD OF PERFORMING A CHEMICAL 4,571,325 2/ 1986 Nikolov et a1. ................... .. 422/191 REACTION AND A REACTOR FOR USE 44,,766352,,953817 182//11998886 VSoalulnhdaerrdst et.. .a..1... ... THEREWITH 4,772,455 9/ 1988 Izumi et a1. ..... .. [75] Inventors: Antonius J. M. van Wingerden, 4,795,618 1/ 1989 Laumen .............. .. Apeldoorn; Andries Q. M. Boon, 5,141,720 8/1992 Malmstrom et a1. . .. Utrecht; John W. Geus, Bilthoven, 5,149,509 9/1992 Fischer et al. .................... .. 422/ 177 all of Netherlands FOREIGN PATENT DOCUMENTS [73] Assignee: Veg-Gasinstituut N.V., Apeldoorn, 3511825 10/1986 Germany . Netherlands 63-20029 1/ 1988 Japan ................................. .. 422/173 [21] Appl. No.: 107,466 8602016 4/1986 WIPO . 9005577 5/1990 WIPO ............................... .. 422/ 173 [22] Filed: Aug. 17, 1993 Primary Examiner—Gary P. Straub Assistant Examiner-Timothy C. Vanoy Related US. Application Data Attorney, Agent, or Firm—Weingarten, Schurgin, [63] Continuation of Ser. No. $79,357, Sep. 7, 1990, aban Gagnebin & Hayes doned. [57] ABSTRACT [30] Foreign Application Priority Data This invention relates to a method of conducting a Sep. 8, 1989 [NL] Netherlands ....................... .. 8902250 chemical reaction in the presence of a heterogeneous catalyst, in which a feedstock is passed through a cata [51] Im. (31.5 ..... .. 1301.1 19/00, 00113 17/16 lytic reactor, said reactor comprising a solid reactor bed [52] us. 01. .................................. .. 423/659; 423/210; with a catalytically active material present thereon, and 423/220; 423/230; 423/573.1; 423/DIG. 6; at least one heat supplying and/or discharging reactor 423/DIG. 13 wall, and in which the catalyst bed consists of elemen [53] Field of Search ................... .. 423/2453, 230, 352, tary particles of material sintered together and to one 423/576, 31, 210, 220, 659, DIG. 13, DIG. 6, side of the wall, there being no sintered material present 573.1; 165/104.12; 422/202, 173, 201 on the other side of the reactor wall, and in which the [56] References Cited maximum distance in meters (x,,,,,;) of any point of the U.S. PATENT DOCUMENTS reactor bed to a nearest heat supplying and/ or discharg ing wall is determined by the following formula: 2,205,409 6/ 1940 Houdry ............................. .. 422/201 3,431,083 3/ 1969 3,617,227 11/1971 2 3,679,372 7/1972 M, M1 h~ATmax 3,929,670 12/1975 ai-a-Z + (aha-21+ a-q 4,101,287 7/1978 4,193,793 3/ 1980 4,423,022 12/1983 4,507,274 3/ 1985 11 Claims, 2 Drawing Sheets ///l’//////////////Z //////////I///l’// U.S. Patent Nov. 22, 1994 Sheet 1 of 2 C) CD LL. CO CD Ll. ///////////////////////////, <[ ‘- <——- .—_ (D u. '///////////// // // // //_////////// 5,366,719 1 2 ide in the production of sulfuric acid, the reaction of NIEI'HOD OF PERFORMING A CHEMICAL sulfur dioxide with hydrogen sul?de in the Claus pro REACTION AND A REACTOR FOR USE cess, the selective oxidation of H28 to S, and the reac THEREWITH tion of carbon monoxide with hydrogen to form meth 5 ane. As thermal energy is generated as these reactions This application is a continuation of application Ser. proceed, the temperature of the reaction mixture will No. 07/579,357, ?led Sep. 7, 1990, now abandoned, rise, and the thermodynamic equilibrium be shifted in an entitled: A METHOD OF PERFORMING A CHEM adverse way, unless the reaction heat generated is rap ICAL REACTION AND A REACTOR FOR USE idly and ef?ciently removed from the reactor. THEREWITH. In endothermic reactions, there may also be a shift of the thermodynamic equilibrium in an undesirable direc FIELD OF THE INVENTION tion, now by absorbing thermal energy. Examples are This invention relates to a method of performing a the methane-steam reforming and the dehydrogenation chemical reaction in the presence of a heterogeneous of ethyl benzene to styrene. Also, the reaction velocity catalyst, in which a feedstock is passed through a cata may become so low that the desired reaction is not lytic reactor. More speci?cally, the invention relates to completed any longer. a method in which the chemical reaction is highly exo In addition to a shift of the thermodynamic equilib thermic or highly endothermic. rium in an undesirable direction, a change in tempera ture may adversely affect the selectivity of catalytic BACKGROUND OF THE INVENTION 20 reactions. In the most current catalytic reactors, use is made of Examples of reactions in which the temperature af a solid catalytic bed. In such a catalyst bed, porous fects selectivity are the production of ethylene oxide bodies are poured or stacked. from ethylene (the undesirable reaction is the formation In order to avoid an undesirably high pressure drop of water and carbon dioxide), the selective oxidation of across the catalyst bed, the bodies or particles used 25 hydrogen sul?de to elemental sulfur (the undesirable preferably have a size of at least 0.3 mm. This minimum reaction is the formation of S02), and the Fischer size of the catalyst bodies is necessary to keep the pres Tropsch synthesis. In all cases the temperature is in sure drop which occurs when a stream of reactants is creased as a result of the generation of reaction heat. passed through the catalyst bed within technically ac When the rise in temperature is not prevented by rap ceptable limits. In addition to the dimension at lower idly removing the reaction heat, the selectivity de limit dictated by the permissible pressure drop, the re creases substantially. quired activity of the catalyst imposes an upper limit Although, as the above examples show, there is a upon the dimensions of the catalytically active particles. great need of a rapid supply or removal of thermal The high activity required for a number of types of energy in catalytic reactors, a solid catalyst bed has a technical catalysts can mostly only be achieved with an 35 poor thermal conductivity. According to the present area of the active phase of 25 to 500 m2 per ml. Surface state of the art, it is virtually impossible to supply ther areas of such an order of magnitude are only possible mal energy to, or remove energy from, a solid catalyst with very small particles, for example, with particles of bed in an efficient manner. Indeed, this is apparent from 0.05 micron. the way in which technical reactions are carried out in As a liquid or gas mixture cannot ?ow through parti 40 solid catalyst beds. cles of such dimensions, the minute primary particles It is possible that, in an exothermic reaction, a rise in must be formed into highly-porous bodies having di temperature only leads to a shift of the thermodynamic mensions of at least about 0.3 mm, which may have a equilibrium in an undesirable direction, without an in large catalytic surface area. An important aim in the tolerable reduction in selectivity. In that case the reac production of technical catalysts is to combine the re 45 tion may be permitted to proceed adiabatically in a solid quired high porosity with a suf?ciently high mechanical catalyst bed; naturally, this is only possible with exo strength. The catalyst bodies must not disintegrate dur thermic reactions. After the passage through the reac ing the ?lling of the reactor and when subjected to tor, the stream of reactants is cooled in a separate heat sudden temperature differences (“thermal shock”). exchanger. As the conversion of the reactants is now Under the conditions of the thermal pre-treatment thermodynamically limited owing to the rise in temper and/or the catalytic reaction to be carried out, almost ature in the reactor, the reactants must be re-reacted all catalytically active materials are rapidly sintered into after cooling. The reaction product may be separated large conglomerates with a negligible small active sur and the reactants recycled through the solid catalyst face area. Generally speaking, therefore, the active bed. This is effected, for example, in the synthesis of component is applied (in ?nely-divided form) to a so 55 ammonia and in the methanol synthesis. If the reaction called carrier. This carrier has the required thermal product cannot be readily separated, the heat exchanger stability and is hardly, if at all, sintered at high tempera should be followed by a second solid-bed reactor with tures. Carriers mostly used are silicon dioxide, alumin heat exchanger. This is the case, for example, in the ium oxide or activated carbon. oxidation of sulfur dioxide to sulfur trioxide. Some A great many technical reactions are characterized 60 times, in order to prevent the emission of harmful com by a more or less large, positive or negative, heat effect. pounds, even a third reactor with heat exchanger is In order that chemical reactions may proceed in the required. If the series-connection of a plurality of reac desired manner, an efficient supply or removal of the tors and heat exchangers is not quite possible, and the reaction heat is indispensable. Thus with some exother separation of the reaction product is not possible either, mic reactions, the thermodynamic equilibrium is shifted 65 the reaction product is sometimes recycled through the in an undesirable direction when the temperature is catalyst bed. In that case, so little of the reactants is increased. Examples are the synthesis of ammonia and added to the circulating reaction product per passage methanol, the oxidation of sulfur dioxide to sulfur triox through the reactor that these are completely con 5,366,719 3 4 verted. As the rise in temperature must then be well example, to reactors in which ?ue gas from large plants controlled, only a small amount can be converted per must be puri?ed, such as in the catalytic removal of pass through the reactor. In cases where the reaction nitrogen oxides from ?ue gas. As a ?ue gas stream is must be carried out at greatly increased pressure, the generally a huge quantity, a proper pressure drop re problems with the supply or removal of reaction heat quires a great deal of mechanical energy. The same are even greater. In the synthesis of ammonia and the applies to the puri?cation of automobile exhaust gases. methanol synthesis, a catalyst bed is used in which reac In that case, too, a high pressure drop is impermissible. tants are injected at different points at a relatively low At the present time, the only possibility of achieving temperature. an acceptable pressure drop, without unduly reducing Such a performance of the process, in which gas the contact with the catalyst, is the use of catalysts streams must be passed through high-pressure reactors applied to a honeycomb. For this purpose almost exclu in a complicated manner, naturally requires high invest sively ceramic honeycombs are used, sometimes re ments. ferred to a “monoliths”, to which the catalytically ac It will be clear that none of the above-discussed tech tive material has been applied. However, these ceramic nical solutions is attractive. Generally speaking, expen honeycombs are very expensive, and their use is there sive equipment is required, while recycling and separa fore not very attractive. tion of reaction products present in low concentration In a variant of the method in which the catalyst is require much energy. This is why, in the cases dis applied to the wall only, monoliths built up from thin cussed, ?uidized beds are often used. In a ?uidized bed, metal plates are used. Such a reactor is made, for exam the transport of thermal energy is much easier. In a 20 ple, by rolling up a combination of corrugated and ?at ?uidized bed, the catalyst to be used must satisfy very thin metal plates and subsequently ?xing this assembly severe demands as regards mechanical strength and by welding. Also, the ?at plates may be stacked to form wear resistance, which is by no means possible with every catalyst. Finally, owing to the inevitable wear, a system with a large number of channels. The catalyst catalyst consumption in a ?uidized bed is relatively is then applied to the wall of the channels thus pro high. Indeed, in many cases it will not be possible to use duced. Here again, with a normal throughput, conver a ?uidized bed. sion is limited, because a relatively large fraction of the There are cases in which neither a ?uidized bed nor reactants passes the catalyst unreacted, or because the an adiabatic reactor can be used. This is especially true reactants require a relatively long residence time in the of highly endothermic reactions and reactions in which reactor for a suf?ciently high conversion to be selectivity is prohibitively decreased when the tempera achieved. ' ture rises. Examples are the methane-steam reforming As stated before, the thermal conduction in a solid and the selective oxidation of ethylene to ethylene ox catalyst bed is poor. This has been attributed to the low ide. In a selective oxidation of ethylene, a very large thermal conductivity of the high-porosity carriers sup heat exchange surface area is applied by using a reactor porting the catalytically active material. This is why with no fewer than 20,000 long tubes. In the methane Kovalanko, O. N. et al, Chemical Abstracts 97 (18) steam reforming process, it is endeavoured to optimize 15l409u, have proposed to improve the thermal con the supply of heat by adapting the dimensions and shape duction by increasing the conductivity of the catalyst of the catalyst bodies. In this latter reaction, too, a large bodies. They did this by using porous metal bodies as number of expensive pipes must be used in the reactor. 40 the catalyst carrier. Now, Satter?eld has already de In certain cases, the reaction can be conducted with a scribed that the thermal conductivity of a stack of po catalyst suspended in a liquid with a suitable boiling rous bodies is determined not so much by the conduc point. The reaction heat is now dissipated by evaporat tivity of the material of the bodies, but by the mutual ing the liquid. In endothermic reactions, thermal energy contacts between the bodies (C. N. Satter?eld, “Mass can be supplied to the reaction system through the liq 45 Transfer in Heterogeneous Catalysis”, MIT Press, uid phase. Technically, however, conducting the cata Cambridge, Mass., USA (1970), page 173). Measure lytic reaction with a catalyst suspended in a liquid phase ments by the present inventors have shown that the is possible in a few cases only. thermal conductivity of catalyst bodies indeed does not It has also been proposed to apply the catalyst exclu substantially affect the transfer of heat in a catalyst bed. sively to the wall of the reactor. This is the case, for In WO-A 86/02016, a reactor is described which example, in the performance of the Fischer Tropsch includes a catalyst carrying reaction bed which consists reaction, in which higher hydrocarbons are produced of sintered metal particles which are in a good heat from a mixture of hydrogen and carbon monoxide. The conducting connection with the reactor wall, which catalyst applied to the wall ensures a good transfer of wall is provided on the outside with sintered metal heat from the catalyst to the environment. One method 55 particles for the discharge of reaction heat. Further proposed for applying the catalyst to the wall is the following. The catalyst is applied to the wall as a Raney more, a change in phase takes place on the outside of the metal, an alloy of the active metal and aluminium. After reactor. It is found that such a reactor system is capable the application, the catalyst is activated by dissolving of realizing a discharge of large amounts of heat, but it the aluminium with lye. The greater part of the reactor 60 has the disadvantage that effective adjustment and/or volume is empty, so that the contact of the reactants control of the reaction is impossible, or very difficult. with the catalytically active surface is poor, and the This appears, inter alia, from the example, which de conversion per pass through the reactor is greatly lim scribes the catalytic combustion of a combustible gas ited. For this reason the reactants must be recycled with a calori?c value of 35,530 k]. This should take through the reactor many times. 65 place at a temperature of 350° C. Owing to the reactor In a number of technically important cases, the pres being cooled with evaporating water (steam produc~ sure drop during the passage of the reactants through tion) at 110° C., however, the entire reactor is cooled to the catalyst bed must be very small. This applies, for 110° C., so that the reaction will fail to proceed. 5,366,719 5 6 There is accordingly a need for a system in which a the heat conductivity of the material used in the solid chemical reaction, in particular one with a substantial state; preferably, this value ranges between 10 and 75%. heat effect, can be carried out in a simple manner. In absolute terms, the heat conductivity ranges between SUMMARY OF THE INVENTION 2 and 250 W/mK. czi is the coefficient of heat transfer in W/mZ/K on the According to the present invention, it has been found reactor side of the heat supplying and/o r discharging that reactions of the type referred to can be excellently reactor wall and equals 6 a, where 6 has a value of carried out in a catalytic reactor in which the reactor between 1.0 and 10 and indicates the increase in heat bed consists of elementary particles of material sintered transfer by sintering the material, and a is the coeffi together and to one side of the reactor wall, but in cient of heat transfer from the reactor bed in the which no sintered metal particles are present on the non-sintered state to the heat supplying and/or dis other side of the reactor wall. If, in such a reactor, the diameter of the reactor bed is selected in relation to the charging reactor wall. The values for 7», (1i and a are heat effects, which differ from reaction to reaction, but determined by the methods indicated in Powell, R. are known and can be calculated depending on reaction W., “Thermal Conductivity Determination by Ther conditions, reactions of the type referred to can be mal Comparator Methods”, Thermal Conductivity, carried out in an optimum manner. Vol. 2, Academic Press, 1969; of Carslaw, H. S., The invention is based on the insight that for a cata Jaeger, J. C.; Conduction of Heat in Solids, Oxford lytic reaction to be carried out on a practical scale re University Press, 2nd Ed., 1959. quires the reaction to proceed within a speci?c tempera ATmax, measured across a radial cross-section through 20 ture range. If heat must be supplied in the case of endo the reactor bed, is the absolute value of the difference thermic reactions and if heat must be discharged in the in temperature between any point in this cross-section case of exothermic reactions, the temperature will re and the nearest heat supplying and/or discharging spectively rise above, and decrease below, this tempera reactor wall, the maximum permissible value of ture range. The present invention makes it possible, which ranges between 1 and 1000K, depending on however, to maintain the optimum temperature by se the type of reaction and the reactor material. The lecting the most suitable reactor system. value for ATM; is determined by the values for a BRIEF DESCRIPTION OF THE DRAWING minimum temperature Tmin and a maximum temper ature Tmax, determined by external factors. The basis Other features and bene?ts of the invention can be of a Tmin and a Tmax may vary greatly; maximum or more clearly understood with reference to the speci?ca minimum temperature to prevent side reactions and tion and the accompanying drawings in which: / or subsequent reactions, freezing of the equilibrium, FIG. 1 schematically illustrates one embodiment of or too low a reaction rate, unduly high pressure drop, the catalyst system according to the present invention; stability, sufficiently high rise in temperature in the and 35 reactor from requirements of the subsequent unit FIG. 2 illustrates the method of applying a catalyti operation, material limitations, condensation of input cally active material to the ?xedly-connected elemen tary bodies. materials or reaction products, etc. a is a form factor determined only by the geometrical DESCRIPTION OF THE PREFERRED shape of the reactor. For a tube and a plate it is 0.25 EMBODIMENT and 0.5, respectively. Generally speaking, a ranges The invention accordingly relates to a method of between these two values. conducting a chemical reaction in the presence of a a’ is a form factor representing the ratio between the heterogeneous catalyst, in which a feedstock is passed area of the reactor cross-section and the heat ex through a catalytic reactor, said reactor comprising a changing circumference of said reactor cross-section solid reactor bed with a catalytically active material 45 multiplied by xmax. Consequently, a’ is only depen present thereon, and at least one heat supplying and/or dent upon the geometry of the reactor. For a tube, a’ discharging reactor wall, and in which the catalyst bed is 0.5 and for a plate 1. In Table 1, values for a and a’ consists of elementary particles of material sintered are listed for a number of reactor geometries. together and to one side of the wall, there being no TABLE 1 sintered material present on the other side of the reactor wall, and in which the maximum distance in meters reactor form a a‘ a'/ a (xmax) of any point of the reactor bed to a nearest heat tube .25 .5 2 supplying and/or discharging wall is determined by the square .29 .5 1.72 following formula: rectangle 55 2 X 1 .45 § 1.48 4 X l .497 4/5 1.61 8 X l .500 8/9 1.78 Z - M, t M, J + 16 X l .500 16/17 1.88 ai-a-Z + plate .5 l 2 In this formula, A is the effective coef?cient of heat The meaning of q in the formula is the maximum conductivity in W/mK of the sintered material. The reaction heat, in W/m3, generated in the reactor bed rate of heat transfer is a relatively important factor in and which must be supplied and/or discharged through the catalyst systems according to the invention. Natu the wall in radial direction. The value of q depends inter rally, the heat conductivity of the overall system, i.e., 65 alia, on the catalyst activity, the load density of the from the reactor wall to within the bed, is partly deter catalyst, the geometry of the catalyst, and the gas com mined by the heat conductivity of the material used. position. For a given reaction or under given reaction Preferably, the heat conductivity is no less than 10% of conditions, q can be determined in a simple manner. 5,366,719 7 8 If it is desired to determine Xmax in relation to the contact with the material of the sintered bodies to pre heat transported in the radial direction per m2 reactor vent undesirable interaction between the material of the wall, the relation can be written as: bodies and the catalytically active component. Suitable metals for use in elementary particles are, among others, nickel, iron, chromium, manganese, va xmax E )t. a? ( ATM“ 1 nadium, cobalt, copper, titanium, zirconium, hafnium, Q ai tin, antimony, silver, gold, platinum, palladium, tung sten, tantalum, and the lanthanides and actinides. The where a, a’, it, ATM,‘ and ai have the above meanings, elementary particles may consist of substantially pure and Q is the heat transported in radial direction per in2 metal or of an alloy of two or more metals, which alloy reactor wall. The magnitude of Q depends on the heat q may contain non-metallic components, such as carbon, produced or required per unit volume and to be dis nitrogen, oxygen, sulfur , silicon, and the like. charged or supplied in the radial direction, and the size According to a different embodiment of the inven of the heat-exchanging surface. tion, the elementary particles consist of ?bres of ?la Q can be simply determined by determining the heat ments, preferably with a diameter of no more than 0.5 ?ux through the outside of the reactor. This heat must mm, more preferably 1-250 um. The materials of which be discharged and/or supplied at the outside of the these particles are made preferably comprise carbon reactor. This can be done in various ways, depending on and metal or metal alloys. the temperature range dictated by the reaction and/or In the present speci?cation, the term “reactor wall” by external factors; for example, by means of gases and 20 means the physical separation between the space where liquids, possibly in a ?uidized bed, irradiation by means the reaction takes place, that is to say, the catalyst bed, of infrared radiators and/or open ?ames, conduction and the space where heat supply and discharge takes through solids, etc. place. This may of course be the outer wall of the cata In the method and the reactor according to this in lyst bed, but also includes, for example, the walls of vention, the elementary bodies forming the solid cata 25 chaimels within the bed, through which heat exchange lyst bed, which may or may not be porous, may consist ?uid can be passed. of all sorts of materials and may be formed in various The wall may consist of the conventional materials ways. A ?rst point of importance is that the particles known for these purposes. These walls may consist of a can be ?xed together, for example by sintering. The single layer, but alternatively, more than one layer is dimensions of the bodies are important, for one thing, used, in which connection it may in particular be advan for the pressure drop across the reactor: when the bod tageous when the surface to which the elementary bod ies are too small, the pressure drop may become too ies must be ?xedly connected enhance the adherence high. Detenninative of the pressure drop across the with the elementary bodies. One example is the use of catalyst bed is the pore structure of the sintered bed. The preferred starting product is, therefore, elementary 35 enamel coating with ceramic elementary bodies. The degree of porosity of the solid catalyst bed ac bodies whose largest dimension is at least 0.3 mm. If desired, smaller basic particles may be used, which cording to the invention may vary within wide limits. during sintering coalesce to form larger bodies, so that This porosity, that is to say, the part of the bed not occupied by ?xedly-connected elementary particles, yet an acceptable pressure drop is obtained. The upper limit of the size is partly determined by the degree of 40 generally ranges between 20 and 90% by volume. The contact between catalyst and reactant(s), while the most suitable value depends on the desired surface area, shape of the bodies may play a role as well. the desired pressure drop and the rate of heat transfer in According to a preferred embodiment of the inven the bed. Preferred values for the porosity range be tion, the catalyst bed is built up from more or less iso tween 40 and 85% by volume. tropic particles, more speci?cally in rather a narrow 45 The rate of heat transfer is a relatively important particle size distribution. When such elementary parti factor in the catalyst systems according to the inven cles are used, a catalyst system with very good proper tion. Naturally, the thermal conductivity of the overall ties is obtained. system, that is to say, from the reactor wall to within the The material of which the elementary particles con bed, is partly determined by the thermal conductivity of sist is preferably metal, but alternatively may be alu the material used. Preferably, the thermal conductivity mina, silica, silica-alumina, zeolite, titanium dioxide, is no less than 10% of that of the employed material in zinc oxide or zirconium oxide, or oxides of a combina the solid state; preferably, this value is between 10 and tion of elements, such as spinel (MgA2O4), mullite (3A1 75%. In absolute terms, the thermal conductivity is 2O3.2Si0;) or corderite (2MgO.2Al2O3.5SiO2), as well preferably between 0.2 and 300 W/mZK. as carbides, nitrides and borides of elements, such as 55 The thermal conductivity depends greatly upon the silicon, tungsten, titanium and vanadium. Metal or thermal conductivity of the elementary bodies used. metal alloys are preferred, because elementary particles Thus, for example, A1203 extrudate has a conductivity consisting of these materials can relatively easily be of 0.32 W/m.K, whereas a sintered body of 316 L has a ?xedly connected together and to the reactor wall by value of 3-12 W/m.K. Powder of 316 L, on the other sintering. The metal or metal alloy may itself be catalyt 60 hand, has a value of 0.55, while solid material has a ically active, or rendered catalytically active by a treat value of 20 W/m.K. Solid copper has a thermal conduc ment, but alternatively, a catalytically active material is tivity of 398 W/m.K. All these values relate to the applied to it. condition at room temperature. At other temperatures, More particularly, it is possible ?rst to apply a cata the absolute value of the ?gures is changed, but their lytically active component to a (highly) porous carrier, 65 mutual ratios remain approximately the same. and attach the thus laden carrier to the metal or alloy The catalyst system according to the present inven surface. This latter may be of importance, if the catalyti tion is, in principle, applicable to any catalytic chemical cally active component must not come into direct reaction, but is more particularly suitable for those reac 5,366,719 9 10 tions which have a strong thermal effect, that is to say, oxidized to form elemental sulfur which by condensa strongly endothermic or exothermic reactions, or reac~ tion is very simple to separate. As such gas mixtures tions in which the selectivity is highly temperature cannot be properly processed in a Claus process, the dependent. In a number of technically important cases, invention is of particularly great importance for these it is desired to employ a high to very high space veloc purposes. ity, whereby a high pressure drop across the reactor is As stated before, the catalyst system according to the not regarded as a great drawback. In the presently-used present invention may be catalytically active of its own, solid-bed reactors, a high pressure drop with the associ or activated by a treatment. It is also very possible, ated high space velocity is not quite possible. If the however, to apply a catalytically active material to the pressure at the reactor input is raised, the catalyst may ?xedly connected elementary bodies. For this purpose, be blown or washed out of the reactor, depending on there is ?rst prepared a dispersion of catalytically active whether the reactants are gaseous or liquid. It is also material, which if desired may be applied to a carrier, in possible that, at a given critical value of the pressure at a liquid, and subsequently this liquid is applied to the the reactor input, “channelling” occurs. In that case, the ?xedly-connected elementary bodies in a suitable man catalyst particles begin to move in a given part of the ner. This can be effected, for example, by evacuating reactor. It is then found that the reactants ?ow almost the bed to which the catalytically active material is to exclusively through that part of the catalyst bed that is be applied, and subsequently sucking the dispersion into in motion. Because, in the reactor according to the the bed, as a result of which the bed is impregnated with present invention, the catalyst particles are much better the catalytically active material. The composition of the ?xed, such a reactor permits using a much higher veloc 20 dispersion and the conditions for making the system are ity of the reactants (and accordingly a much higher preferably so selected that the viscosity of the impreg pressure drop across the reactor). Under certain condi nating liquid is increased after the impregnation. The tions, this may be of great advantage. Another impor fact is that in this way the liquid phase can be removed tant advantage of ?xing the catalyst bodies within the from the dispersion without substantially disturbing the reactor is apparent in case dust is deposited on the cata 25 distribution of the catalytically active material. More lyst bed. In current solid-bed reactors, the catalyst bed over, in this way, a better distribution of the catalyti is clogged. The reactor must be opened and the dust cally active material over the ?xedly-connected ele layer removed. In reactors according to the present mentary bodies is obtained. A number of methods are invention, a high-pressure gas pulse may be sent conceivable for increasing the viscosity of the liquid. A through the reactor in a direction opposite to that of the 30 ?rst method is constituted by cooling it below its solidi stream of reactants. This pressure pulse blows the dust ?cation point, so that the entire mass becomes solid. By from the catalyst bed, and so clogging can be prevented using a vacuum the system can then be freeze dried. without opening the reactor, which is very attractive Another posssibility, and one which is preferred, is from a technical point of view. With solid-bed catalysts constituted by incorporating a minor proportion of agar according to the state of the art, this is not possible: the 35 or other material having a comparable activity, in the catalyst bodies will be blown out of the catalyst bed dispersion, so that the dispersion may be introduced into along with the dust layer. the system at elevated temperature and the system may With non-sintered material, owing to the fact that the subsequently be ?xed by simple cooling. Subsequently, shape of the material particles does not conform to that the liquid may be removed under vacuum or otherwise, of the reactor wall, the reactor bed has a high porosity 40 and the agar removed at elevated temperature by pyrol at the wall. As a consequence, little catalyst is present at ysis. The suitable proportion of agar is mainly deter this location, and relatively much less feedstock will be mined by the wish that the liquid must be capable of reacted. This effect is enhanced still further as the high becoming suf?ciently viscous, or even solid. Suitable porosity gives low pressure losses, and the feedstock concentrations range between 0.05 and 1.0% by weight. will preferentially ?ow along the wall. Sintering, how 45 The catalyst systems to be used in the method accord ever, improves the connection with the wall, and as a ing to the present invention will be explained in greater result the porosity is in the order of the bed material not detail with reference to the accompanying drawings. present at the wall. In addition, where the catalyst is FIG. 1 schematically illustrates one embodiment of applied after the reactor has been ?lled and sintered, the catalyst system according to the present invention, catalyst will also be deposited on the wall. The result of 50 with increasing magni?cation from left to right. In the these effects is that slip-through along the wall is much left-hand drawing, the system as such is illustrated, with less, and the overall length of the reactor may be the reactants entering the system from the bottom, and shorter. Thus the pressure drop remains low, and there the products leaving the system at the top. The heat is an alternative for honeycombs. produced leaves the system in accordance with the The invention is particularly suitable for conducting 55 arrows shown at the left, and is discharged using a highly exothermic or endothermic catalytic reactions. heat-exchange medium now shown. As an example of such a reaction, the oxidation of meth The middle drawing is a magni?cation of the drawing ane will be described. As an example of a reaction in described above, and illustrates in detail a piece of the which the selectivity is greatly determined by the tem reactor wall with ?xedly-connected elementary bodies. perature, the selective oxidation of hydrogen sul?de 60 In it, 1 indicates the matrix of ?xedly-connected metal will be taken. In this case, the discharge of thermal particles, 2 the reactor wall, and 3 a supplementary energy is of great importance, as above a temperature of heat-exchange material. about 300° C., the oxidation of sulfur vapour to the The right-hand drawing illustrates a still higher mag undesirable sulfur dioxide is going to proceed. The use ni?cation, and shows the location of the catalytically of a catalyst system according to the present invention 65 active particles 4 on the metal 1. makes it possible for gas streams with a hydrogen sul FIG. 2 illustrates in the form of a sketch the method ?de content of, for example, 10% by volume, to be very of applying a catalytically active material to the ?xedly efficiently puri?ed. The hydrogen sul?de is selectively connected elementary bodies. In the ?gure, 1 indicates 5,366,719 11 12 the ?xedly-connected elementary particles and 2 the and Ah, with Ah being the reaction heat released per reactor wall. This wall forms part of a reactor which is converted pressure unit of component c,-. provided at the top and bottom with stubs for the sup 4. In view of the composition of the feedstock and the ply and discharge of materials. These stubs are provided temperature range, in conjunction with corrosion with valves 7, 8. The reactor is surrounded with a jacket resistance, a material can be selected which the 9 for heat exchanging ?uid, for example water. This reactor is to consist of. jacket is provided with stubs 10 and 11. Inlet 5 is pro . By means of the data from the supplier of the sin vided with a reservoir 12 for liquid which can be intro tered material, a particle size and a pore size distri duced into the system. For the introduction of catalyti bution can be selected. This is correlated to the cally active material into the system, the procedure is permissible pressure drop and the required resi then as follows: dence time in the reactor material in order that a given conversion be achieved. The length of the Valve 7 is closed and stub 6 is connected to a vacuum pump, while valve 8 is open. Contained in reservoir 12 reactor and the average velocity at which the feed is a dispersion of the catalytically active material to be stock flows through the reactor are determined simultaneously. These will depend on the tempera introduced into the system in a liquid. As soon as the ture, which will generally not be constant, the vacuum in the reactor is suf?cient, valve 8 is closed. By permitted pressure drop, and the required resi opening valve 7, the dispersion is sucked into the sys dence time. This step often requires an integration tem. As soon as there is sufficient dispersion in the sys over the reactor length to arrive at an accurate tem, the vacuum can be fully released, and reservoir 12 determination of length, feedstock velocity, parti removed. The system is cooled through the jacket until cle size and pore size distribution. the liquid is sufficiently viscous or solidi?ed. Subse 6. The diameter or thickness of an element of the quently, the whole is dried in vacuo. reactor (for example a plate or a tube) can be calcu Example 1 lated by means of the formula here given. 25 7. The cooling or heating means to be used must be Starting from a given reaction in which heat must be selected on the basis of the amount of heat to be discharged or supplied, the design of a reactor will discharged or supplied and the required tempera comprise the following steps. ture range. 1. Determination of the reaction, the desired tempera 8. The number of reactor elements can be determined ture range, the absolute pressure, the inlet concen 30 by means of the amount of feedstock to be pro trations and the required conversion and the cessed and the amount per reactor element, which amount of feedstock. can be calculated by means of 6. . Determination of the kinetic data of the catalyst used in the reaction in question. This can be done Example 2 either in a trial reactor with sintered metal on 35 20 g 'y-Al2O3 (A1 4172, 265 m2/g , pore volume 1.14 which the catalyst has been deposited, or in a trial ml/ g) was suspended in 750 ml deionized water of 30° reactor with just catalysts deposited on ceramic C. The pH was adjusted to 5 by means of concentrated material. In the latter case, a correction will have HNO3. 1.95 g EDTA (ethylene diamine tetraacetic to be made for the changed loading of the catalyst. acid) was dissolved in 50 ml deionized water by drop All this will lead to a relation which describes or 40 ping concentrated ammonia into it, taking care that the sufficiently accurately approaches the reaction rate pH did not decrease below 4. 2.69 g La(N03)3.6H2O within the ranges of temperature and partial pressures (corresponding to an ultimate load of 5% by weight of of the reactants. Generally speaking this relation will be La2O3) was dissolved in 5 ml deionized water and care as follows: fully dropped into the EDTA solution. The pH was 45 maintained between 4 and 7 by adding dilute ammonia dPCi dropwise. The ultimate solution was poured in the aque dz ous suspension of 'y—Al2O3. The pH was re-adjusted to 5 by adding dilute HNO3 dropwise. The suspension was vigorously stirred for one hour, and the pH was kept 50 constant by injecting dilute HNO3 below the surface of dPCi the liquid. After one hour, the suspension was ?ltered 111 and washed twice with 25 ml deionized water. The carrier material was then dried at 60° C. for 16 hours. is the change of component i per time unit and per The dried carrier material was calcined in the air at a volume unit of reactor bed. 55 temperature of 550° C. for 5.5 hours to convert the -r is the contact period between the reactants and lanthanum complex into the oxidic form. the catalyst. The carrier material eventually contains 3% by T is the temperature at which the reaction takes weight of La2O3. 15 g of this stabilized carrier was place. suspended in 750 ml deionized water of 30° C. PC,- is the partial pressure of component i 60 5.16 g Cu(N03)2.3H2O was dissolved in 50 ml deion P is the pressure of the system ized water and added to the suspension. The suspension It is the number of reactants involved in the reac was vigorously. stirred while N; was insuf?ated below tion. the surface of the liquid. With concentrated HNO3, the 3. q then follows from the product pH was adjusted to 4. By injecting a 0.5M NaOH solu 65 tion (0.3 ml/m in) below the surface of the liquid, the pH was increased to 12. After 16 hours, the catalyst was dPCi dt ?ltered and washed twice with 25 ml deionized water. The catalyst was dried at 60° C. for 23 hours. 5,366,719 13 14 This catalyst was subsequently ground and sieved If both catalysts, that is to say, a conventional catalyst with a 25 um mesh sieve. The powder was suspended in with non-sintered ceramic material and one applied to water to form a 10 wt. % mixture, and the pH was sintered metal (aluminium) according to the present adjusted to 5. Then 0.25% by weight of agar (pro invention, are introduced into tubular reactors and the analysi) was added to this mixture. It was heated on a reaction proceeds at 300° C. then by means of the data water bath to 90° C. and then maintained at this temper of Table 2, which also lists the results, the tube diameter ature for a minimum period of 2 minutes to allow the can be calculated. aTmax is set at 25° C. in connection agar to dissolve completely. with two undesirable effects. At a lower temperature Thereafter a steel pipe containing a porous metal (316 the equilibrium conversion is unduly low. At higher L) body with pores of a size greater than 100 um, was temperatures, the subsequent reaction starts, in which S heated in a water bath of at least 90° C. is oxidized to form 80;. This porous metal body had been previously obtained by sintering elementary particles essentially consisting TABLE 2 of metal powder (316 L) with a particle size of about According to 500 pm together and to the wall. During the heat treat Unit Conventional this invention ment, the porous material was evacuated to 15 mm Hg. Arm, K 25 25 Subsequently, the above mixture containing the cata q w/m3 9 09 ed W/m2K 59 500 lyst, while still warm, was let into the pipe with porous A W/mK 0.39 65 material. After the suspension had been let into the a (tube) _ 0.25 0.25 porous body, the whole was maintained at a tempera 20 a’ (tube) - 0.5 0.5 ture of at least 90° C. for such a long time that the sus Km, m 3.2 - 10-4 0.025 Q w/m2 1440 1 1250 pension appeared at the bottom of the porous body. Thereafter the porous body thus impregnated was cooled to room temperature. This was followed by a The diameter of 0.64 mm which results for the con waiting period of 2 hours to ensure that the agar had 25 ventional case is so small that a reactor cannot be de gelled the suspension. Thereafter all suspension not signed in this way. The only alternative is, therefore, to present in the body was removed. Subsequently the connect free adiabatic reactors in series, where the gas body was dried overnight in a vacuum of substantially enters at a temperature of 200° C. and where, as a result less than the vapour pressure of water at room tempera of the reaction, the temperature rises to 300° C. After ture (15 torr). To accelerate the drying process, the tube each adiabatic reactor, a heat exchanger must be con may be heated in vacuo below the melting point of the nected, in which the temperature is again lowered to solidi?ed suspension (up to maximally 40° C.). Finally, 200° C. the agar was burnt by heating the porous metal body in Example 4 air to above 450° C. With a throughflow resulting in a pressure drop of 1 35 A conventional ethylene oxidation reactor consists of atmosphere, less than 1% of the impregnated catalyst 20,000 tubes with a diameter of 0.02 in. As the gas is was blown out. blown through it at a high velocity (20 m/sec), the Measurements at a space velocity of 6000/hour coefficient of heat transfer is mainly determined by this showed that the activity of the catalyst for the combus velocity. The value for ai can then be estimated to be tion of natural gas had hardly decreased as a result of 40 1000 W/mZK. 7t has been estimated to be 0.39 W/mK. the impregnation here described. By means of the formula with which Xmax can be calculated, so conversely, the quotient Example 3 Selective oxidation of H28 to elemental sulfur. For the manufacture of the reactor, a steel pipe was 45 = 6.91 - 10—5, used. In it, metal particles of an iron-chromium alloy were introduced, with the composition being 80% iron which is not known from the open literature, can be and 20% chromium. The metal particles had a size of calculated. 900 to 2000 pm. The alloy powder poured into the tube By means of the values for sintered metal consisting was densitied by vibration and then sintered in a hydro 50 of aluminium with ai=500 W/m2K and k=65 W/mK, gen atmosphere at 1200° C. for 10 hours. This resulted xmax can be calculated for a reaction according to this in a porous metal body rigidly anchored in the tube. invention as being 0.0567 111. The reactor was then heated at 800° C. for 16 hours, The diameter of these tubes should then be 11.3 cm. while air was passed through it. As a result of this treat This means that, instead of 20,000 tubes, only 630 are ment, the activity of the surface for the formation of 55 required. sulfur dioxide disappeared. We claim: Subsequently, in the manner described in Example 2, 1. A method of conducting a chemical reaction in the the catalytically active component was applied to the presence of a heterogeneous catalyst, said method com pre-treated alloy. The active component consisted of prising the steps of: (IL-A1203, to which 5% of Fe2O3/Cr2O3 with an Fe/ Cr 60 a. selecting a heterogeneous catalyst for use in a cata ratio of 9/1 had been applied. The speci?c area of the lytic reactor; a-Al2O3 was 6.5 in2 per g. This active component was b. forming said catalytic reactor so as to include at loaded into the reactor in a proportion of 600 kg per m3 least one heat supplying and/or discharging reac catalyst bed volume. tor wall having an inside surface and an outside If 16 m3/sec of a gas containing 5% H25 is oxidized 65 surface and de?ning a reactor volume; by reaction by means of the above catalyst to convert c. forming a solid reactor bed comprising elementary the H25 into S, the conventional system can be com particles of carrier material sintered together to pared with a reactor according to the present invention. form sintered material and having said heteroge
Description: