Table Of ContentUS 20120322867Al
(19) United States
(12) Patent Application Publication (10) Pub. No.: US 2012/0322867 A1
Hughes et al. (43) Pub. Date: Dec. 20, 2012
(54) METHODS OF TREATING AN OVERWEIGHT Dec. 4, 2008, provisional application No. 61/119,891,
OR OBESE SUBJECT ?led on Dec. 4, 2008, provisional application No.
61/119,888, ?led on Dec. 4, 2008, provisional appli
(76) Inventors: Thomas E- Hughes, Concord, MA (US); cation No. 61/119,891, ?led on Dec. 4, 2008, provi
James E-Vath, Lynn?eld, MA (US) sional application No. 61/275,688, ?led on Aug. 3,
2009, provisional application No. 61/260,194, ?led on
(21) Appl. No.: 13/568,800 Nov. 11,2009.
(22) Filed: Aug. 7, 2012 Publication Classi?cation
Related US. Application Data (51) Int- Cl
A61K 31/336 (2006.01)
(63) Continuation of application No. 13/133,060, ?led on A611) 3/04 (200601)
0“ 27’ 2011’ ?led as aPPllcatlon NO- PCT/US2009/ (52) us. Cl. ..................................................... .. 514/475
066816 on Dec. 4, 2009.
(60) Provisional application No. 61/119,881, ?led on Dec. (57) ABSTRACT
4, 2008, provisional application No. 61/119,875, ?led The invention generally relates to methods of treating an
on Dec. 4, 2008, provisional application No. 61/ 119, overweight or obese subject, and treating overWeight- or obe
884, ?led on Dec. 4, 2008, provisional application No. sity-related conditions. In certain embodiments, the invention
61/119,886, ?led on Dec. 4, 2008, provisional appli provides a method of treating an overweight or obese subject
cation No. 61/119,872, ?led on Dec. 4, 2008, provi including administering a MetAP2 inhibitor in Which the
sional application No. 61/119,877, ?led on Dec. 4, amount administered does not substantially modulate angio
2008, provisional application No. 61/119,885, ?led on genesis.
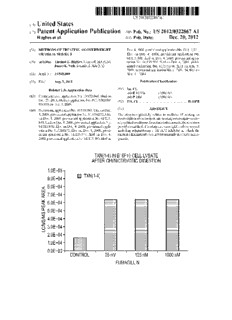
TXN(1-4) IN B16F10 CELL LVSATE
AFTER CHVMOTRVOTIC DIGESTION
1.0E+O5 -
E1 TXN(1-4)
9.0E+04 -
8.0E+04 -
7.0E+O4 -
LPAC/ERIVAEIS/KAMS
6.0E+O4 -
5.0E+O4 -
4.0E+O4 -
3.0E+04 -
2.0E+O4 -
1.0E+O4 -
0.0E+00
CONTROL 25 nM
FUIVIAGILLIN
Patent Application Publication Dec. 20, 2012 Sheet 1 0f 13 US 2012/0322867 A1
TXN(1-4) IN B16F10 CELL LVSATE
AFTER CHVIVIOTRVOTIC DIGESTION
1.0E+05—
E1 TXN(1-4)
9.0E+04
%
< 7.0E+04—
LgI)J 5.0E+04
Eg 3.0E+04—
2.0E+04—
1.0E+04—
0.0E+OO
CONTROL ' 25nM ' 125nM ' 1000nM
FUMAGILLIN
FIG. 1
Patent Application Publication Dec. 20, 2012 Sheet 2 0f 13 US 2012/0322867 A1
N-ACETYL TXN(1-4) IN B16F10 CELL LVSATE
AFTER CHVMOTRVOTIC DIGESTION
1.0E+04
E N-ACETYL TXN(1-4)
9.0E+03
8.0E+03
111111111
111111111 11111111
11 111 11 11111111
111111111 111 1111
7.0E+03 111111111111111111 1111111111111111
1/ 111 11 11111111
111111111 11111111
LPAC/ERIVAEIS/KAMS 111111111 11111111
111111111 11111111
6.0E+03 111111111 111111111 11111111
11111111 111111111 11111111
111111111 111111111 11111111
111111111 111111111 11111111
111111111 11 111 11 11111111
111111111 111111111 11111111
5.0E+03 111111111 111111111 11111111
111111111 111111111 11111111
1 11111 11 111 11 11111111
111111111 111111111 11111111
1111/1111 111111111 11111111
111111111 111111111 11111111
4.0E+03 111111111 111111111 11111111
111111111 111111111 11111111
111111111 11 111 11 11111111
111111111 111111111 11111111
111111111 111111111 11111111
3.0E+03 111111111111111111 1111 1111111 11111 1111111111111111
111111111 111111111 11111111
111111111 111111111 11111111
111111111 111111111 11111111
1111/1111 11 111 11 11111111
2.0E+03 111111111/11111111 111111111111111111 1111111111111111
111111111 111111111 11111111
111111111 111111111 11111111
111111111 111111111 11111111
111111111 11 111 11 11111111
1.0E+03 111111111111111111 1111 1111111 11111 1111111111111111
111111111 111111111 11111111
111 11111 11 111 11 11111111
111111111 111111111 11111111
11111 111111111 1111111
0.0E+00 111111111 111111111 11111111
CONTROL 25 nM 125 nM 1000 nM
FUMAGILLIN
FIG. 2
Patent Application Publication Dec. 20, 2012 Sheet 3 0f 13 US 2012/0322867 A1
DN 29/4/09
SH3BGRL+F
SHSBGRL
.CUS-1+F a-FLAG H2
TXN+F
GUS-1
TXN 30sec
*N 1
250 —
148- _
36 —
22
16
FIG. 3
Patent Application Publication Dec. 20, 2012 Sheet 4 0f 13 US 2012/0322867 A1
TRANS
1u0m0
1u0M 1M0M
1MM
600 —
250 —
148—
98—
50
36—
22—
16 —
TbN
FIG. 4
Patent Application Publication Dec. 20, 2012 Sheet 5 0f 13 US 2012/0322867 A1
(‘l/b3“) awaunamoaam-vua
0
s s s ' ,_ g
5 2‘ E
;_ B» >
E’ (m/baw) XXX AiiVzl
\ _ _ g oamaaisawow
200-................
I
5
i i |
O O 0 0
LO 0 L0
Patent Application Publication Dec. 20, 2012 Sheet 7 0f 13 US 2012/0322867 A1
EPIDIDYIVIAL FAT
PERI-RENAL FAT
LIVER
AGE-IVIATCHED HIGH FAT HIGH FAT
LEAN DIET DIET +
ZGN 201
FIG. 7
Patent Application Publication Dec. 20, 2012 Sheet 8 0f 13 US 2012/0322867 A1
Ll
00
L9
LL
Ir
<lr | |
LO 00 N C)
(6) Cl‘v'd 1w ‘IVEINOLIEIEICIOEILEIEI
Description:Aug 7, 2012 HIGH FAT. HIGH FAT. LEAN. DIET. DIET +. ZGN 201. FIG. 7 13/133,060 ?led
Oct. 27, 201 1, Which is anational stage ?ling Page 24