INChO 2008 Chemistry Solution from India Indian National Chemistry Olympiad PDF
Preview INChO 2008 Chemistry Solution from India Indian National Chemistry Olympiad
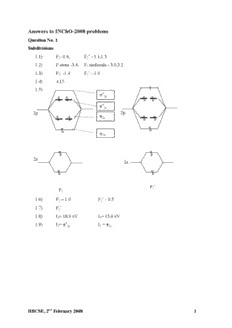
Answers to INChO-2008 problems Question No. 1 Subdivisions 1.1) F -1.6, F - 1.1,1.3 2 2 1.2) F atom -3.4, F molecule - 3.0,3.2 2 1.3) F : -1.4 F : -1.9 2 2 1.4) 4.15 1.5) 2p 2p 2p 2p 2p 2p 2 s 2s F F2 2 1.6) F – 1.0 F - 0.5 2 2 1.7) F 2 1.8) I = 18.9 eV I = 15.6 eV 1 2 1.9) I = I = 1 2p 2 2p HBCSE, 2nd February 2008 1 Question No. 2 Subdivisions 2.1) a) Z-5-methyl hex-2-en-1-al b) Z-2-methyl-1-phenyl hept-1-en-6-yne 2.2) B C T.S.1 T.S.2 2.3) y g r en Int e ee r F product Reaction Coordinate 2.4) i) E ii) D iii) E 2.5) a) iv b) iii c) ii d) v e) i H 2.6) O O H OO OO H O H O H CH C C CH 2.7) D. 2 3 O CH2COOH E. CO OH F. O O O O OH OH 2.8) G. Br CH CH CH CH Br 2 2 Br CH CH CH CH 2 2 H. Br HBCSE, 2nd February 2008 2 I. J. CH Br CH Br 2 2 H R Br H R Br S R H Br Br H CH Br CH Br 2 2 O O O 2.9) O O O O O K O 2.10) O L O Question No. 3 Subdivisions 3.1) i) aromatic iv) yes ii) aromatic v) acidic iii) vi) a) 3.2) I < III < II COOH COOH 3.3) K L HO H C O 7 3 NO NO 2 2 COCl OH M N N(C H ) H C O 2 5 2 7 3 NO O 2 O O C OC H 3 7 O C OC H O 3 7 P N(C H ) NH2 2 5 2 N(C H ) NO 2 5 2 2 HBCSE, 2nd February 2008 3 3.4) B r NO2 Br NO2 O O OMe OMe Br + Br Br 3.5) A. B. C. D. + N N Br 3.6) iv. Question No. 4 Subdivisions 4.1) 8.17 10-8 bar 4.2) 304 K 4.3) 641.02 K 4.4) Yes the reaction will proceed towards NOCl 4.5) Rate = k [NO]2 [Cl ] 2 4.6) E = 98.82 kJ / mol a 4.7) Mechanism I and II both are possible. 4.8) Extent of the reaction = 0.1 4.9) Extent of the reaction at completion = 0. 195 Question No. 5 Subdivisions 5.1) a. 5.2) b. 5.3) X Solid phase 1 X Solid –Liquid equilibrium phase 2 X Liquid – Gas equilibrium phase 3 X Gas Phase 4 HBCSE, 2nd February 2008 4 5.4) gas p Liquid –gas m T0 e equilibrium T Liquid T Solid-liquid m equilibrium solid Time 5.5) b 5.6) Volume will increase on melting 5.7) Single Phase system called Supercritical Fluid 5.8) c p p 1 p 5.9) 1 1 1 2 1 p p p or 1 1 2 1 p p 1 2 1 p / p 1 1 2 5.10) 56 5.11) a. nA =A n [A] n KC 2 b. C K 1 D n C 2 HBCSE, 2nd February 2008 5 Question No. 6 Subdivisions 6.1) P P P P 6.2) P (s) + 3 NaOH + 3H O PH (g) + 3 H PO Na+ 4 2 3 2 2 2 6.3) a. H Na+ H H H or P O or H P O P + P 2Na H O ONa O O O ONa O ONa b. hypophosphite-reducing agent phosphate –reducing agent c. They are reducing agents due to presence of P-H bond and lower oxidation state of P 6.4) Ca (PO ) F + 5H SO 3H PO + 5CaSO + HF 5 4 3 2 4 3 4 4 6.5) O O P O P O O or O O O 6.6) O 5P04 Ogs PoPf COaOO. O P O POOP O O O 6.7) PCl + O 2Cl PO O 3 2 3 POCl +3EtOH O P(OEt) + 3HCl 3 3 6.8) 3s 3p 3d Ground state Excited State Cl Cl P Cl Or trigonal bipyramidal Cl Cl HBCSE, 2nd February 2008 6 6.9) 3PCl + 3NH Cl (Cl PN) + 12 HCl 5 4 2 3 Cl Cl P N N Cl Cl P P Cl Cl N HBCSE, 2nd February 2008 7 Question No. 7 Subdivisions 7.1) Co3+ : 3d6 4S0 7.2) A Pink: [Co(NH ) .H O]Cl : Pentaamine aqua cobalt(III)chloride 3 5 2 3 B Purple: [CoCl(NH ) ]Cl : Pentaamine chlorocobalt(III)chloride 3 5 2 7.3) Co3+ = 3d6 3d 4s 4p XX XX XX XX XX XX d2sp3 hybridisation Octahedral 7.4) a. e g t 2g Co3+=d6 b. diamagnetic. [Co(NH ) ]2+ = Co2+ [Co(NH ) ]3+ = Co3+ 7.5) 3 6 3 6 Loss of electron ea sy HBCSE, 2nd February 2008 8 NH NH 3 3 7.6) NH Cl 3 Cl Co NH3 Cl Co NH3 Cl Cl Cl NH 3 Facial Meridional 7.7) Facial isomer - one peak due to ammonia (with all similar environments) Meridional isomer – two peaks 7.8) Cl O O O O Co O Co O Cl O O Cl Cl Inactive plane of symmetry Chiral Question No. 8 Subdivisions 8.1) Fe(s) + 2OH(aq) FeO(s) + H O(l) + 2e 2 Ni O (s) + H O(l) + 2e 2NiO(s) + 2OH(aq) 2 3 2 Fe(s) + Ni O (s) FeO(s) + 2NiO(s) 2 3 8.2) iii. 8.3) E0 = 0.83633 V cell E = 0.80283 V cell 8.4) Ag(s)+ Fe3+(aq) = Fe2+(aq) + Ag+(aq) Fe(s) + 2Ag+(aq) = Fe2+(aq) + 2Ag(s) 8.5) K 2.97 [Fe3]0.01 HBCSE, 2nd February 2008 9 Question No. 9 Subdivisions 9.1) H NCH COOH 2 2 9.2) i) Nylon COOH ii) H N CH NH + (CH ) COOH 2 2 2 2 6 9.3) Nature Charge Peptide Acidic Basic Neutral Positive Negative Zero Gly-Leu-Val X X Leu-Trp-Lys-Gly-Lys X X Arg-Ser-Val X X 9.4) a) + HN-CH-COO 3 CH 2 S S CH CH CH CH 2 6 5 2 3 + + + 2H3N-CH-COO + H3N-CH-COO + 2H3N-CH-COO b) + HN-CH-COO 3 SH CH 2 CH 2 S (H) + 2HN-CH-COO 3 S CH 2 + HN-CH-COO 3 9.5) i) 4 H O COO ii) + H3N C C NH C H CH COOCH CH C H 2 3 2 6 5 HBCSE, 2nd February 2008 10
