Hexahydropyrano [3, 4-d][1, 3] Thiazin-2-Amine Compounds PDF
Preview Hexahydropyrano [3, 4-d][1, 3] Thiazin-2-Amine Compounds
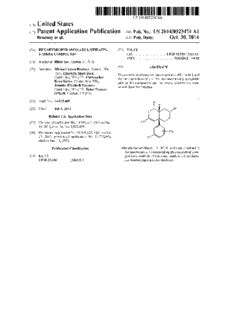
US 20140323474A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0323474 A1 Brodney et al. (43) Pub. Date: Oct. 30, 2014 (54) HEXAHYDROPYRANO[3,4-D][1,3]THIAZIN- (52) US. Cl. 2-AMINE COMPOUNDS CPC .................................. .. C07D 513/04 (2013.01) USPC ........................................ .. 514/2242; 544/48 (71) Applicant: P?zer Inc., Groton, CT (US) (72) Inventors: Michael Aaron Brodney, Newton, MA (57) ABSTRACT (CUSE Egzablslt: “£1?er g?ck’ h The present invention provides compounds of Formula I, and am n ge’ ( )’ [1st er the tautomers thereof, and the pharmaceutically acceptable ($63172: flgrgiilgaiftl?og’algiegs)’ salts of the compounds and tautomers, Wherein the com ’ d h th t tu Cambridge, MA (US); Brian Thomas poun S ave e S we re O’Neill, Haddam, CT (US) l (21) Appl.No.: 14/325,409 (22) Filed: Jul. 8, 2014 Related US. Application Data (62) Division of application No. 14/101,462, ?led on Dec. 10, 2013, noW Pat. No. 8,822,456. (60) Provisional application No. 61/895,623, ?led on Oct. 25, 2013, provisional application No. 61/735,660, ?led on Dec. 11,2012. Publication Classi?cation Wherein the variables R1, R2, R3 , R4 and X are as de?ned in the speci?cation. Corresponding pharmaceutical com (51) Int. Cl. positions, methods of treatment, methods of synthesis, C07D 513/04 (2006.01) and intermediates are also disclosed. US 2014/0323474 A1 Oct. 30, 2014 HEXAHYDROPYRANO [3,4-D] [1,3] THIAZIN-2 risk factors of AD. In particular, there is a higher risk of AMINE COMPOUNDS sporadic AD in patients with Type 2 diabetes and AD patients are more prone to Type 2 diabetes (Butler, Diabetes, 53:474 481, 2004.). Recently, it has also been proposed that AD FIELD OF THE INVENTION should be reconsidered as Type 3 Diabetes (de la Monte, J. Diabetes Sci. Technol., 2008; 2(6): 1 101-1 1 13). Of special [0001] The present invention relates to small molecule inhibitors of [3-site amyloid precursor protein (APP) Cleaving interest is the fact that AD and Type 2 diabetes share common pathogenic mechanisms and possibly treatments (Park SA., J. Enzyme 1 (BACE1). In particular, this invention relates to inhibiting the production of A-beta peptides that can contrib Clin. Neurol., 2011; 7: 10-18; Raffa, Br. J. Clin. Pharmacol ute to the formation of neurological deposits of amyloid pro 2011/71:3/365-376). Elevated plasma levels ofA[3, the prod tein, which may be applicable in the treatment of Alzheimer’ s uct of BACE activities, were recently associated with hyper Disease (AD) and other neurodegenerative and/ or neurologi glycemia and obesity in humans (see Meakin et al., Biochem cal disorders in mammals. In addition, this invention is 1., 2012, 441(1):285-96.; Martins, Journal of Alzheimer’s related to the treatment of diabetes and obesity in mammals, Disease, 8 (2005) 269-282). Moreover, increasedA[3 produc including humans. tion prompts the onset of glucose intolerance and insulin resistance in mice (Cozar-Castellano, Am. J. Physiol. Endo crinol. Metab., 302:E1373-E1380, 2012.; Delibegovic, Dia BACKGROUND OF THE INVENTION betologia (2011) 54:2143-2151). Finally, it is also suggested [0002] Dementia results from a wide variety of distinctive that circulating AB could participate in the development of pathological processes. The most common pathological pro atherosclerosis in both humans and mice (De Meyer, Athero cesses causing dementia are Alzheimer’s disease (“AD”), sclerosis 216 (2011) 54-58; Catapano, Atherosclerosis 210 cerebral amyloid angiopathy (“CM”) and prion-mediated dis (2010) 78-87; Roher, Biochimica et Biophysica Acta 1812 eases (see, e.g., Haan et al., Clin. Neurol. Neurosurg., 1990, (2011)1508-1514). 92(4):305-310; Glenner et al., J. Neurol. Sci., 1989, 94: 1-28). [0006] Therefore, it is believed that BACE1 levels may play AD is a progressive, neurodegenerative disorder character a critical role in glucose and lipid homoeostasis in conditions ized by memory impairment and cognitive dysfunction. AD of chronic nutrient excess. Speci?cally, BACE1 inhibitors affects nearly half of all people past the age of 85, the most rapidly growing portion of the United States population. As may be potentially useful for increasing insulin sensitivity in such, the number of AD patients in the United States is skeletal muscle and liver as illustrated by the fact that reduc expected to increase from about 4 million to about 14 million tion in BACE1 decreases body weight, protects against diet by 2050. induced obesity and enhances insulin sensitivity in mice (see Meakin et al., Biochem. J. 2012, 441(1):285-96). Of equal [0003] The accumulation of amyloid-[3 (AB peptides) is interest is the identi?cation of LRPl as a BACE1 substrate believed to be one of the underlying causes of Alzheimer’s and the potential link to atherosclerosis (Strickland, Physiol. Disease (AD), which is the most common cause of cognitive Rev., 88: 887-918, 2008; Hyman, J. Biol. Chem., Vol. 280, decline in the elderly (Hardy & Allsop, Trends Pharmacol No. 18, 17777-17785, 2005). Sci., 1991; 12(10):383-8; Selkoe, Behav. Brain Res., 2008; 192(1): 1 06- 1 3). AB, the major protein constituent of amyloid [0007] Likewise, inhibition of BACE2 is proposed as a plaques, is derived from sequential cleavage of the type I treatment of Type 2 diabetes with the potential to preserve and integral membrane protein, amyloid precursor protein (APP) restore [3-cell mass and stimulate insulin secretion in pre by two proteases, [3- and y-secretase. Proteolytic cleavage of diabetic and diabetic patients. (WO201 1/ 020806). BACE2 is APP by the [3-site APP cleaving enzymes (BACE1 and a [3-cell enriched protease that regulates pancreatic [3 cell BACE2) generates a soluble N-terminal ectodomain of APP function and mass and is a close homologue of BACE1. (sAPP[3) and the C-terminal fragment C99. Subsequent Pharmacological inhibition of BACE2 increases [3-cell mass cleavage of the membrane bound C99 fragment by the and function, leading to the stabilization of Tmem27. (See y-secretase liberates the various AB peptide species, of which Esterhazy et al., Cell Metabolism 2011, 14(3): 365-377). It is A[340 andA[342 are the most predominant forms (Vassar et al, suggested that BACE2 inhibitors are useful in the treatment J. Neurosci., 2009; 29(41): 12787-94; Marks & Berg, Neuro and/or prevention of diseases associated with the inhibition of chem. Res., 2010; 35:181-210). Therefore, limiting the gen BACE2 (e.g., Type 2 diabetes, with the potential to preserve eration of AB directly through inhibition of BACE1 is one of and restore [3-cell mass and stimulate insulin secretion in the most attractive approaches for the treatment of AD, as pre-diabetic and diabetic patients) (WO2011/020806). BACE1 inhibitors could effectively inhibit the formation of all predominant AB peptides. [0008] Aminodihydrothiazine or thioamidine compounds are described in US2009/0082560, WO 2009/091016 and [0004] In addition, it has been determined that BACE1 knock-out mice had markedly enhanced clearance of axonal WO 2010/038686 as useful inhibitors of the [3-secretase and myelin debris from degenerated ?bers, accelerated enzyme. Co-pending PCT application, PCT/IB2012/054198, axonal regeneration, and earlier reinnervation of neuromus ?led by P?zer Inc on Aug. 17, 2012, also describes aminodi cular junctions compared with littermate controls. These data hydrothiazine compounds that are useful inhibitors of the suggest BACE1 inhibition as a therapeutic approach to accel [3-secretase enzyme. The present invention is directed to erate regeneration and recovery after peripheral nerve dam novel thioamidine compounds and their use in the treatment age. (See Farah etal., J. Neurosci., 2011, 31(15): 5744-5754). of neurodegenerative diseases, including AD, as well as the [0005] Insulin resistance and impaired glucose homoeosta treatment of metabolic diseases and conditions such as dia sis are important indicators of Type 2 diabetes and are early betes and obesity. US 2014/0323474 A1 Oct. 30, 2014 SUMMARY OF THE INVENTION [0025] (5) The pharmaceutical composition described herein for inhibiting BACE activity for the therapeutic and/ or [0009] The present invention relates to: prophylactic treatment of diseases and disorders character [0010] (l) A compound of Formula I, ized by elevated [3-amyloid levels, including diabetes or type 2 diabetes; [0026] (6) The pharmaceutical composition described I herein for increasing insulin sensitivity in skeletal muscle and liver in a mammal, including humans; R2 [0027] (7) The pharmaceutical composition described herein for treating and/ or preventing obesity. [0028] (8) A compound or tautomer thereof or pharmaceu tically acceptable salt of said compound or tautomer, or the solvate thereof, Wherein the compound is selected from Examples 1-20, further described in Table l; [0029] (9) Methods of inhibiting BACE enzyme activity, by administering a therapeutically effective amount of a thioa midine compound of any of the embodiments of Formula I or Wherein a pharmaceutically acceptable salt thereof, and a pharmaceu [0011] R1 is hydrogen or methyl, Wherein said methyl is tically acceptable carrier, to a mammal or a patient in need optionally substituted With one to three substituents indepen thereof. dently selected from halogen or methoxy; [0030] (10) Methods for treating conditions or diseases of [0012] R2 is C1_6 alkyl, i(C(R5)2)mi(C3_6 cycloalkyl), the central nervous system and neurological disorders in i(C(R5)2)m5 i(C6-10ary1)$ i(C(R5)2)mi(5- to lo'mem' Which the [3-secretase enzyme is involved (such as migraine; bered heteroaryl) or i(C(R5)2)tiOR6; Wherein said alkyl, epilepsy; Alzheimer’s disease; Parkinson’s disease; brain cycloalkyl, aryl or heteroaryl moieties are optionally substi injury; stroke; cerebrovascular diseases (including cerebral tuted With one to three substituents independently selected arteriosclerosis, cerebral amyloid angiopathy, hereditary from halogen, C1_6 alkyl, 4CH2F, 4CHF2, iCF3, iCN or cerebral hemorrhage, and brain hypoxia-ischemia); cognitive iOR7; disorders (including amnesia, senile dementia, HIV-associ [0013] R3 is *(C(R5)2)m, *(CN) Or *(C(R5)2)n* ated dementia, Alzheimer’s disease, Huntington’s disease, (NHR7); Lewy body dementia, vascular dementia, drug-related [0014] R4 is independently halogen, C1_6 alkyl or C1_6 dementia, tardive dyskinesia, myoclonus, dystonia, delirium, alkoxy; Wherein said alkyl or alkoxy is optionally substituted Pick’s disease, Creutzfeldt-Jacob disease, HIV disease, With one to three ?uoro; Gilles de la Tourette’s syndrome, epilepsy, muscular spasms [0015] R5 at each occurrence is independently hydrogen or and disorders associated With muscular spasticity or weak Cl_3 alkyl, Wherein said alkyl is optionally substituted With ness including tremors, and mild cognitive impairment (“MCI”); mental de?ciency (including spasticity, Down syn one to three halogen; drome and fragile X syndrome); sleep disorders (including [0016] R6 is hydrogen, C1_6 alkyl or i(C(R6)2)ni(C6_ 10 aryl), Wherein said alkyl and aryl are optionally substituted hypersomnia, circadian rhythm sleep disorder, insomnia, parasomnia, and sleep deprivation) and psychiatric disorders With one to three substituents independently selected from halogen, Cl_3 alkyl, 4CH2F, 4CHF2, iCF3, 4CN or such as anxiety (including acute stress disorder, generalized anxiety disorder, social anxiety disorder, panic disorder, post iOH; traumatic stress disorder, agoraphobia, and obsessive-com [0017] R7 for each occurrence is hydrogen or C1_6 alkyl, pulsive disorder); factitious disorder (including acute hallu Wherein said alkyl is optionally substituted With one to three cinatory mania); impulse control disorders (including substituents independently selected from halogen or iCl_6 alkoxy; compulsive gambling and intermittent explosive disorder); mood disorders (including bipolar I disorder, bipolar II dis [0018] m for each occurrence is 0, l or 2; order, mania, mixed affective state, major depression, chronic [0019] n for each occurrence is l or 2; and depression, seasonal depression, psychotic depression, sea [0020] tis l or 2; and sonal depression, premenstrual syndrome (PMS) premen [0021] x is 0, 1,2 or 3; strual dysphoric disorder (PDD), and postpartum depres or a tautomer thereof or a pharmaceutically acceptable salt of sion); psychomotor disorder; psychotic disorders (including said compound or tautomer. schizophrenia, schizoaffective disorder, schizophreniform, [0022] (2) A pharmaceutical composition comprising a and delusional disorder); drug dependence (including nar compound of the invention, or a tautomer thereof or a phar cotic dependence, alcoholism, amphetamine dependence, maceutically acceptable salt of said compound or tautomer, cocaine addiction, nicotine dependence, and drug Withdrawal or a solvate thereof, and a pharmaceutically acceptable syndrome); eating disorders (including anorexia, bulimia, vehicle, diluent or carrier; binge eating disorder, hyperphagia, obesity, compulsive eat [0023] (3) The pharmaceutical composition described ing disorders and pagophagia); sexual dysfunction disorders; herein for inhibiting production of amyloid-[3 protein and for urinary incontinence; neuronal damage disorders (including inhibiting beta-site amyloid precursor protein cleaving ocular damage, retinopathy or macular degeneration of the enzyme 1 (BACEl); eye, tinnitus, hearing impairment and loss, and brain edema), [0024] (4) The pharmaceutical composition described nerve injury treatment (including accelerating regeneration herein for treating a neurodegenerative disease and, in par and recovery after peripheral nerve damage) and pediatric ticular, Alzheimer’s Disease; psychiatric disorders (including attention de?cit disorder, US 2014/0323474 A1 Oct. 30, 2014 attention de?cit/hyperactive disorder, conduct disorder, and DETAILED DESCRIPTION OF THE INVENTION autism) in a mammal, preferably a human, comprising [0038] The present invention may be understood more administering to said mammal a therapeutically effective readily by reference to the following detailed description of amount of a compound of Formula I or pharmaceutically exemplary embodiments of the invention and the examples acceptable salt thereof. The compounds of Formula I may included therein. It is to be understood that this invention is also be useful for improving memory (both short-term and not limited to speci?c methods of synthesis, which may of long-term) and learning ability. The text revision of the fourth course vary. It is also to be understood that the terminology edition of the Diagnostic and Statistical Manual of Mental used herein is for the purpose of describing particular Disorders (DSM-IV-TR) (2000, American Psychiatric Asso embodiments only and is not intended to be limiting. ciation, Washington, DC.) provides a diagnostic tool for [0039] In this speci?cation and in the claims that follow, identifying many of the disorders described herein. The reference will be made to a number of terms that shall be skilled artisan will recognize that there are alternative nomen de?ned to have the following meanings: clatures, nosologies, and classi?cation systems for disorders [0040] The term “alkyl” refers to a linear or branched-chain described herein, including those as described in the DMS saturated hydrocarbyl substituent (i.e., a substituent obtained IV-TR, and that terminology and classi?cation systems from a hydrocarbon by removal of a hydrogen); in one evolve with medical scienti?c progress; embodiment from one to six carbon atoms; and in another [0031] (11) Methods for treating a neurological disorder embodiment from one to four carbon atoms; and in another (such as migraine; epilepsy; Alzheimer’s disease; Parkin embodiment one to three carbon atoms. Non-limiting son’s disease; Niemann-Pick type C; brain injury; stroke; examples of such substituents include methyl, ethyl, propyl cerebrovascular disease; cognitive disorder; sleep disorder) (including n-propyl and isopropyl), butyl (including n-butyl, or a psychiatric disorder (such as anxiety; factitious disorder; isobutyl, sec-butyl and tert-butyl), pentyl, isoamyl, hexyl and impulse control disorder; mood disorder; psychomotor dis the like. order; psychotic disorder; drug dependence; eating disorder; [0041] In some instances, the number of carbon atoms in a and pediatric psychiatric disorder) in a mammal, preferably a hydrocarbyl substituent (i.e., alkyl, cycloalkyl, etc.) is indi human, comprising administering to said mammal a thera cated by the pre?x “Cx-Cy-” or “Cx_y”, wherein x is the mini peutically effective amount of a compound of Formula I or mum and y is the maximum number of carbon atoms in the pharmaceutically acceptable salt thereof; substituent. Thus, for example, “C 1-C6-alkyl” or “C l_6 alkyl” [0032] (12) Methods for the treatment (e.g., delaying the refers to an alkyl substituent containing from 1 to 6 carbon progression or onset) of diabetes or diabetes-related disorders atoms. Illustrating further, C3-C6-cycloalkyl or C3_6-cy including Type 1 and Type 2 diabetes, impaired glucose tol cloalkyl refers to saturated cycloalkyl containing from 3 to 6 erance, insulin resistance, hyperglycemia, and diabetic com carbon ring atoms. plications such as atherosclerosis, coronary heart disease, [0042] The term “alkoxy” refers to a straight chain satu stroke, peripheral vascular disease, nephropathy, hyperten rated alkyl or branched chain saturated alkyl bonded through sion, neuropathy, and retinopathy; an oxygen atom. Non-limiting examples of such alkoxy [0033] (13) Methods for the treatment of obesity co-mor groups are methoxy, ethoxy, propoxy, isopropoxy, butoxy, bidities, such as metabolic syndrome. Metabolic syndrome isobutoxy, tertiary butoxy, and pentoxy. includes diseases, conditions or disorders such as dyslipi [0043] The term “aryl” means a carbocyclic aromatic sys demia, hypertension, insulin resistance, diabetes (e.g., Type 2 tem containing one, two or three rings wherein such rings diabetes), coronary artery disease and heart failure. For more may be fused. If the rings are fused, one of the rings must be detailed information on metabolic syndrome, see, e.g., Zim fully unsaturated and the fused ring(s) may be fully saturated, met, P. Z. et al., “The Metabolic Syndrome: Perhaps an Etio partially unsaturated or fully unsaturated. The term “fused” logic Mystery but Far From a MythiWhere Does the Inter means that a second ring is present (i.e., attached or formed) national Diabetes Federation Stand?,” Medscape Diabetes & by having two adjacent atoms in common (i.e., shared) with Endocrinology, 7(2), (2005); and Alberti, K. G. et al., “The the ?rst ring. The term “fused” is equivalent to the term Metabolic SyndromeiA New Worldwide De?nition,” Lan “condensed”. The term “aryl” embraces aromatic radicals cet, 366, 1059-62 (2005); such as phenyl, naphthyl, tetrahydronaphthyl, indanyl, biphe [0034] (14) Methods for the treatment of nonalcoholic fatty nyl, benzo[b][l,4]ovain-3(4H)-onyl, 2,3-dihydro-1H inde liver disease (NAFLD) and hepatic insulin resistance; nyl, and l,2,3,4-tetrahydronaphthalenyl. [0035] (15) Combination therapies wherein the compounds [0044] The term “cycloalkyl” refers to a nonaromatic ring of this invention may also be used in conjunction with other that is fully hydrogenated and exists as a single ring. pharmaceutical agents for the treatment of the diseases, con Examples of such carbocyclic rings include cyclopropyl, ditions and/ or disorders described herein. Therefore, methods cyclobutyl, cyclopentyl, and cyclohexyl. of treatment that include administering compounds of the [0045] The term “halo” or “halogen” refers to ?uorine present invention in combination with other pharmaceutical (which may be depicted as iF), chlorine (which may be agents are also provided; depicted as 4C1), bromine (which may be depicted as iBr), [0036] All patents, patent applications and references or iodine (which may be depicted as fl). referred to herein are hereby incorporated by reference in [0046] The term “heteroaryl” refers to an aromatic ring their entirety. structure containing from 5 to 6 ring atoms in which at least [0037] Other features and advantages of this invention will one of the ring atoms is a heteroatom (i.e., oxygen, nitrogen, be apparent from this speci?cation and the appendent claims or sulfur), with the remaining ring atoms being independently which describe the invention. It is to be understood that both selected from the group consisting of carbon, oxygen, nitro the foregoing and the following detailed description are gen, and sulfur. Examples of heteroaryl substituents include exemplary only and are not restrictive of the invention as 6-membered ring substituents such as pyridyl, pyrazyl, pyri claimed. midinyl, and pyridaZinyl; and 5-membered ring substituents US 2014/0323474 A1 Oct. 30, 2014 such as triazolyl, imidazolyl, furanyl, thiophenyl, pyrazolyl, heteroaryl. The heteroatoms for this invention are selected oxazolyl, isoxazolyl, thiazolyl, 1,2,3-, 1,2,4-, 1,2,5-, or 1,3, from nitrogen, oxygen and sulfur. 4-oxadiazolyl and isothiazolyl. In a group that has a het [0050] If substituents are described as “independently” eroaryl sub stituent, the ring atom of the heteroaryl sub stituent having more than one variable, each instance of a sub stituent that is bound to the group may be one of the heteroatoms, or is selected independent of the other from the list of variables it may be a ring carbon atom. Similarly, if the heteroaryl available. Each substituent therefore may be identical to or substituent is in turn substituted with a group or substituent, different from the other substituent(s). the group or substituent may be bound to one of the heteroa [0051] As used herein, the term “Formula I” may be here toms, or it may be bound to a ring carbon atom. The term inafter referred to as a “compound(s) of the invention,” “the “heteroaryl” also includes pyridyl N-oxides and groups con present invention,” and “compound of Formula I.” Such terms taining a pyridine N-oxide ring. Further examples include are also de?ned to include all forms of the compound of furyl, thienyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl, tria Formula I, including hydrates, solvates, isomers, crystalline Zolyl, tetrazolyl, isoxazolyl, isothiazolyl, oxadiazolyl, thia and non-crystalline forms, isomorphs, polymorphs, tau diazolyl, pyridinyl, pyridaZinyl, pyrimidinyl, pyraZinyl, pyri tomers and metabolites thereof. For example, the compounds din-2(lH)-onyl, pyridaZin-2(1H)-onyl, pyrimidin-2(lH) of the invention, or pharmaceutically acceptable salts thereof, onyl, pyraZin-2(1H)-onyl, imidazo[ l ,2-a]pyridinyl, pyrazolo may exist in unsolvated and solvated forms. When the solvent [l,5-a]pyridinyl, 5,6,7,8-tetrahydroisoquinolinyl, 5,6,7,8 tetrahydroquinolinyl, 6,7-dihydro-5H-cyclopenta[b] or water is tightly bound, the complex will have a well de?ned stoichiometry independent of humidity. When, how pyridinyl, 6,7-dihydro-5H-cyclopenta[c]pyridinyl, l,4,5,6 ever, the solvent or water is weakly bound, as in channel tetrahydrocyclopenta[c]pyrazolyl, 2,4,5,6 solvates and hygroscopic compounds, the water/ solvent con tetrahydrocyclopenta[c]pyrazolyl, 5 , 6-dihydro -4H-pyrrolo tent will be dependent on humidity and drying conditions. In [l,2-b]pyrazolyl, 6,7-dihydro-5H-pyrrolo[l,2-b][1,2,4] triazolyl, 5,6,7,8-tetrahydro-[l ,2,4]triazolo[l ,5-a]pyridinyl, such cases, non-stoichiometry will be the norm. 4,5,6,7-tetrahydropyrazolo[l,5-a]pyridinyl, 4,5,6,7-tetrahy [0052] The compounds of the invention may exist as clath dro-lH-indazolyl and 4,5,6,7-tetrahydro-2H-indazolyl. The rates or other complexes. Included within the scope of the heteroaryl can be further substituted as de?ned herein. invention are complexes such as clathrates, drug-host inclu sion complexes wherein the drug and host are present in [0047] Examples of single-ring heteroaryls and heterocy stoichiometric or non-stoichiometric amounts. Also included cloalkyls include furanyl, dihydrofuranyl, tetrahydrofuranyl, are complexes of the compounds of the invention containing thiophenyl, dihydrothiophenyl, tetrahydrothiophenyl, pyrro two or more organic and/or inorganic components, which lyl, isopyrrolyl, pyrrolinyl, pyrrolidinyl, imidazolyl, isoimi may be in stoichiometric or non-stoichiometric amounts. The dazolyl, imidazolinyl, imidazolidinyl, pyrazolyl, pyrazolinyl, resulting complexes may be ionized, partially ionized, or pyrazolidinyl, triazolyl, tetrazolyl, dithiolyl, oxathiolyl, non-ionized. For a review of such complexes, see J. Pharm. oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, thiazolinyl, Sci., 64(8), 1269-1288 by Haleblian (August 1975). isothiazolinyl, thiazolidinyl, isothiazolidinyl, thiaoxadiaZ olyl, oxathiazolyl, oxadiazolyl (including oxadiazolyl, 1,2,4 [0053] The compounds of the invention have asymmetric carbon atoms. The carbon-carbon bonds of the compounds of oxadiazolyl, 1,2,5-oxadiazolyl, or 1,3,4-oxadiazolyl), pyra nyl (including 1,2-pyranyl or l,4-pyranyl), dihydropyranyl, the invention may be depicted herein using a solid line ( pyridinyl, piperidinyl, diaZinyl (including pyridaZinyl, pyri ), a solid wedge (-_— ), or a dotted wedge ( midinyl, piperaZinyl, triaZinyl (including s-triaZinyl, as-tri """"'"l ). The use ofa solid line to depict bonds to asymmetric aZinyl and v-triaZinyl), ovainyl (including 2H-l ,2-ovainyl, carbon atoms is meant to indicate that all possible stereoiso 6H-l,3-ovainyl, or 2H-l,4-ovainyl), isovainyl (including mers (e.g., speci?c enantiomers, racemic mixtures, etc.) at o-isovainyl or p-isovainyl), oxazolidinyl, isoxazolidinyl, that carbon atom are included. The use of either a solid or dotted wedge to depict bonds to asymmetric carbon atoms is oxathiaZinyl (including 1,2,5-oxathiaZinyl or 1,2,6-oxathi aZinyl), oxadiaZinyl (including 2H-l,2,4-oxadiaZinyl or meant to indicate that only the stereoisomer shown is meant to 2H-l ,2,5-oxadiaZinyl), morpholinyl. be included. It is possible that compounds of Formula I may contain more than one asymmetric carbon atom. In those [0048] The term “heteroaryl” also includes fused ring sys compounds, the use of a solid line to depict bonds to asym tems having one or two rings wherein such rings may be metric carbon atoms is meant to indicate that all possible fused, wherein fused is as de?ned above. It is to be understood stereoisomers are meant to be included. For example, unless that if a carbocyclic or heterocyclic moiety may be bonded or stated otherwise, it is intended that the compounds of For otherwise attached to a designated substrate through differing mula I can exist as enantiomers and diastereomers or as ring atoms without denoting a speci?c point of attachment, racemates and mixtures thereof. The use of a solid line to then all possible points are intended, whether through a car depict bonds to one or more asymmetric carbon atoms in a bon atom or, for example, a trivalent nitrogen atom. For compound of Formula I and the use of a solid or dotted wedge example, the term “pyridyl” means 2-, 3- or 4-pyridyl, the to depict bonds to other asymmetric carbon atoms in the same term “thienyl” means 2- or 3-thienyl, and so forth. compound is meant to indicate that a mixture of diastere omers is present. [0049] In some instances, the number of atoms in a cyclic substituent containing one or more heteroatoms (i.e., het [0054] Stereoisomers of Formula I include cis and trans eroaryl or heterocycloalkyl) is indicated by the pre?x “x- to isomers, optical isomers such as R and S enantiomers, dias y-membered”, wherein x is the minimum and y is the maxi tereomers, geometric isomers, rotational isomers, conforma mum number of atoms forming the cyclic moiety of the tional isomers, and tautomers of the compounds of the inven substituent. Thus, for example, “5- to 6-membered het tion, including compounds exhibiting more than one type of eroaryl” refers to a heteroaryl containing from 5 to 6 atoms, isomerism; and mixtures thereof (such as racemates and dias including one or more heteroatoms, in the cyclic moiety of the tereomeric pairs). Also included are acid addition or base US 2014/0323474 A1 Oct. 30, 2014 addition salts wherein the counterion is optically active, for suitable for human consumption. Pharmaceutically accept example, D-lactate or L-lysine, or racemic, for example, DL able salts are particularly useful as products of the methods of tartrate or DL-arginine. the present invention because of their greater aqueous solu [0055] When any racemate crystallizes, crystals of two dif bility relative to the parent compound. For use in medicine, ferent types are possible. The ?rst type is the racemic com the salts of the compounds of this invention are non-toxic pound (true racemate) referred to above Wherein one homo “pharmaceutically acceptable salts.” Salts encompassed geneous form of crystal is produced containing both Within the term “pharmaceutically acceptable salts” refer to enantiomers in equimolar amounts. The second type is the non-toxic salts of the compounds of this invention, Which are racemic mixture or conglomerate Wherein two forms of crys generally prepared by reacting the free base With a suitable tal are produced in equimolar amounts each comprising a organic or inorganic acid. single enantiomer. [0059] Suitable pharmaceutically acceptable acid addition [0056] The compounds of Formula I may exhibit the phe salts of the compounds of the present invention Whenpossible nomenon of tautomerism and are regarded as compounds of include those derived from inorganic acids, such as hydro the invention. For example, the compounds of Formula I may chloric, hydrobromic, hydro?uoric, boric, ?uoroboric, phos exist in several tautomeric forms, including the 2-amino phoric, metaphosphoric, nitric, carbonic, sulfonic, and sulfu dihydrothiaZine form, Ia, and the 2-imino -tetrahydrothiaZine ric acids, and organic acids such as acetic, benzenesulfonic, form, Ib. All such tautomeric forms, and mixtures thereof, are benzoic, citric, ethanesulfonic, fumaric, gluconic, glycolic, included Within the scope of compounds of Formula I. Tau isothionic, lactic, lactobionic, maleic, malic, methane tomers exist as mixtures of a tautomeric set in solution. In sulfonic, tri?uoromethanesulfonic, succinic, toluenesulfonic, solid form, usually one tautomer predominates. Even though tartaric, and tri?uoroacetic acids. Suitable organic acids gen one tautomer may be described, the present invention erally include, for example, aliphatic, cycloaliphatic, aro includes all tautomers of the compounds of Formula I and matic, araliphatic, heterocyclic, carboxylic, and sulfonic salts thereof. Examples of tautomers are described by the classes of organic acids. compounds of Formula Ia' and lb' and, collectively and [0060] Speci?c examples of suitable organic acids include generically, are referred to as compounds of Formula I. acetate, tri?uoroacetate, formate, propionate, succinate, gly colate, gluconate, digluconate, lactate, malate, tartrate, cit rate, ascorbate, glucuronate, maleate, fumarate, pyruvate, la' aspartate, glutamate, benzoate, anthranilate, stearate, salicy late, p-hydroxybenzoate, phenylacetate, mandelate, embonate (pamoate), methanesulfonate, ethanesulfonate, benzenesulfonate, pantothenate, toluenesulfonate, 2-hy droxyethanesulfonate, sufanilate, cyclohexylaminosul fonate, algenate, [3-hydroxybutyrate, galactarate, galactur onate, adipate, alginate, butyrate, camphorate, cam phorsulfonate, cyclopentanepropionate, dodecylsulfate, gly coheptanoate, glycerophosphate, heptanoate, hexanoate, nicotinate, 2-naphthalesulfonate, oxalate, palmoate, pecti nate, 3-phenylpropionate, picrate, pivalate, thiocyanate, and undecanoate. [0061] Furthermore, Where the compounds of the invention carry an acidic moiety, suitable pharmaceutically acceptable salts thereof may include alkali metal salts, e.g., sodium or potassium salts; alkaline earth metal salts, e.g., calcium or magnesium salts; and salts formed With suitable organic ligands, e.g., quaternary ammonium salts. In another embodi ment, base salts are formed from bases Which form non-toxic salts, including aluminum, arginine, benzathine, choline, diethylamine, diolamine, glycine, lysine, meglumine, ola mine, tromethamine and Zinc salts. [0057] The compounds of this invention may be used in the [0062] Organic salts may be made from secondary, tertiary form of salts derived from inorganic or organic acids. or quaternary amine salts, such as tromethamine, diethy Depending on the particular compound, a salt of the com lamine, N,N'-dibenzylethylenediamine, chloroprocaine, cho pound may be advantageous due to one or more of the salt’s line, diethanolamine, ethylenediamine, meglumine (N -meth physical properties, such as enhanced pharmaceutical stabil ylglucamine), and procaine. Basic nitrogen-containing ity in differing temperatures and humidities, or a desirable groups may be quaternized With agents such as lower alkyl (Cl-C6) halides (e.g., methyl, ethyl, propyl, and butyl chlo solubility in water or oil. In some instances, a salt of a com pound also may be used as an aid in the isolation, puri?cation, rides, bromides, and iodides), dialkyl sulfates (e. g., dimethyl, and/ or resolution of the compound. diethyl, dibutyl, and diamyl sulfates), long chain halides (e. g., decyl, lauryl, myristyl, and stearyl chlorides, bromides, and [0058] Where a salt is intended to be administered to a patient (as opposed to, for example, being used in an in vitro iodides), arylalkyl halides (e.g., benzyl and phenethyl bro context), the salt preferably is pharmaceutically acceptable. mides), and others. The term “pharmaceutically acceptable salt” refers to a salt [0063] In one embodiment, hemisalts of acids and bases prepared by combining a compound of Formula I With an acid may also be formed, for example, hemisulfate and hemical Whose anion, or a base Whose cation, is generally considered cium salts. US 2014/0323474 A1 Oct. 30, 2014 [0064] Also Within the scope of the present invention are [0069] The term “therapeutically effective amount” means so-called “prodrugs” of the compound of the invention. Thus, an amount of a compound of the present invention that (i) certain derivatives of the compound of the invention that may treats or prevents the particular disease, condition, or disor have little or no pharmacological activity themselves can, der, (ii) attenuates, ameliorates, or eliminates one or more When administered into or onto the body, be converted into symptoms of the particular disease, condition, or disorder, or the compound of the invention having the desired activity, for (iii) prevents or delays the onset of one or more symptoms of example, by hydrolytic cleavage. Such derivatives are the particular disease, condition, or disorder described herein. referred to as “prodrugs.” Further information on the use of [0070] The term “treating”, as used herein, unless other prodrugs may be found in “Pro-drugs as Novel Delivery Wise indicated, means reversing, alleviating, inhibiting the Systems, Vol. 14, ACS Symposium Series (T. Higuchi andV. progress of, delaying the progression of, delaying the onset Stella) and “Bioreversible Carriers in Drug Design,” Perga of, or preventing the disorder or condition to Which such term mon Press, 1987 (ed. E. B. Roche, American Pharmaceutical applies, or one or more symptoms of such disorder or condi Association). Prodrugs in accordance With the invention can, tion. The term “treatment”, as used herein, unless otherWise for example, be produced by replacing appropriate function indicated, refers to the act of treating as “treating” is de?ned alities present in the compounds of any of Formula I With immediately above. The term “treating” also includes adju certain moieties known to those skilled in the art as “pro vant and neo-adjuvant treatment of a subject. For the avoid moieties” as described, for example, in “Design of Prodrugs” ance of doubt, reference herein to “treatment” includes ref by H. Bundgaard (Elsevier, 1985). erence to curative, palliative and prophylactic treatment, and [0065] The present invention also includes isotopically to the administration of a medicament for use in such treat labeled compounds, Which are identical to those recited in ment. [0071] In one embodiment of compounds of Formula I, R3 Formula I, but for the fact that one or more atoms are replaced by an atom having an atomic mass or mass number different is i(C(R5)2)mi(CN); m is 0 or 1; and R5 at each occurrence from the atomic mass or mass number usually found in is hydrogen; or a tautomer thereof or a pharmaceutically nature. Examples of isotopes that can be incorporated into acceptable salt of said compound or tautomer. compounds of the present invention include isotopes of [0072] In another embodiment of compounds of Formula I, hydrogen, carbon, nitrogen, oxygen, sulfur, ?uorine and chlo R3 is i(C(R5)2)ni(NHR7); n is 1; and R5 at each occurrence rine, such as 2H, 3H, 13C, 11C, 14C, 15N, 18O, 17O, 32P, 35S, is hydrogen; or a tautomer thereof or a pharmaceutically 1 8F, and 3 6Cl, respectively. Compounds of the present inven acceptable salt of said compound or tautomer. tion, prodrugs thereof, and pharmaceutically acceptable salts [0073] In another further embodiment of compounds of of said compounds or of said prodrugs Which contain the Formula I, R2 is C1_6 alkyl, optionally substituted With one to aforementioned isotopes and/ or other isotopes of other atoms three ?uoro, or i(C(R5)2)tiOR6; Whereint is 1; R5 at each are Within the scope of this invention. Certain isotopically occurrence is hydrogen, and R6 is Cl_3 alkyl, optionally sub labeled compounds of the present invention, for example stituted With one to three ?uoro, or i(C(R5)2)ni(C6_ l O aryl), those into Which radioactive isotopes such as 3H and 14C are Wherein said aryl is optionally substituted With one to three incorporated, are useful in drug and/or substrate tissue distri sub stituents independently selected from halogen, Cl_6 alkyl, bution assays. Tritiated, i.e., 3H, and carbon-14, i.e., l4C, 4CH2F, iCHF2, 4CF3, iCN or iOH; or a tautomer isotopes are particularly preferred for their ease of prepara thereof or a pharmaceutically acceptable salt of said com tion and detectability. Further, substitution With heavier iso pound or tautomer. topes such as deuterium, i.e., 2H, can afford certain therapeu [0074] In a further embodiment of compounds of Formula tic advantages resulting from greater metabolic stability, for I, R6 is i(C(R5)2)ni(C6_ 10 aryl), said aryl is phenyl option example increased in vivo half-life or reduced dosage ally substituted With substituents independently selected requirements and, hence, may be preferred in some circum from halogen, C1_6 alkyl, 4CH2F, 4CHF2, 4CF3, iCN or stances. Isotopically labeled compounds of Formula I of this 40H; n is 1; and R5 at each occurrence is hydrogen; or a invention and prodrugs thereof can generally be prepared by tautomer thereof or a pharmaceutically acceptable salt of said carrying out the procedures disclosed in the Schemes and/or compound or tautomer. in the Examples and Preparations below, by substituting a [0075] In another embodiment, R2 is i(C(R5)2)mi(C3_6 readily available isotopically labeled reagent for a non-isoto cycloalkyl) or i(C(R5)2)m-(5- to 10-membered heteroaryl), pically labeled reagent. Wherein said cycloalkyl or heteroaryl is optionally substituted With halogen or C1_6 alkyl optionally substituted With one to [0066] As used herein, “eating disorders” refer to illnesses three ?uoro; m is 0; and R5 at each occurrence is hydrogen; or in Which the patient suffers disturbances in his/her eating behaviors and related thoughts and emotions. Representative a tautomer thereof or a pharmaceutically acceptable salt of examples of obesity-related eating disorders include overeat said compound or tautomer. ing, bulimia, binge-eating disorder, compulsive dieting, noc [0076] In further embodiments, R4 is independently ?uoro, chloro, methyl, ethyl, propyl, methoxy or ethoxy, Wherein turnal sleep-related eating disorder, pica, Prader-Willi syn drome, and night-eating syndrome. said methyl, ethyl and propyl groups are optionally substi tuted With one to three ?uoro; and x is 0, 1 or 2; or a tautomer [0067] “Patient” refers to warm-blooded animals such as, thereof or a pharmaceutically acceptable salt of said com for example, guinea pigs, mice, rats, gerbils, cats, rabbits, pound or tautomer. dogs, cattle, goats, sheep, horses, monkeys, chimpanzees, and [0077] In yet another embodiment, R1 is hydrogen; R2 is humans. C 16 alkyl, C3_6 cycloalkyl, 5-membered heteroaryl or [0068] The term “pharmaceutically acceptable” means the 4CH24OR6; Wherein said alkyl is optionally substituted substance or composition must be compatible, chemically With one F; R3 is 4CN or 4CH2i(NHR7); R4 is indepen and/ or toxicologically, With the other ingredients comprising dently halogen or C1_6 alkoxy; R6 is hydrogen, C1_6 alkyl, or a formulation, and/ or the mammal being treated thereWith. 4CH2-phenyl; R7 is C1_6 alkyl optionally substituted With US 2014/0323474 A1 Oct. 30, 2014 one to three sub stituents independently selected from halogen [0083] In another embodiment of a compound of Formula I or C 16 alkoxy; and X is 0, l, or 2; or a tautomer thereof or a is represented by a compound of Formula Ib pharmaceutically acceptable salt of said compound or tau tomer. [0078] In one embodiment, a compound of Formula I is represented by a compound of Formula Ia Wherein R1, R2, R4 and R7 are de?ned as described above; or a tautomer thereof or a pharmaceutically acceptable salt of said compound or tautomer. [0084] In another embodiment, R7 for each occurrence is methyl optionally substituted With one to three ?uoro; or a tautomer thereof or a pharmaceutically acceptable salt of said compound or tautomer. In another embodiment, R2 is Cl_3 alkyl, optionally substituted With one to three ?uoro; R4 is Wherein R1, R2, and R4 are de?ned as described above, or a independently ?uoro, chloro or methoxy; and X is 0, l or 2; or tautomer thereof or a pharmaceutically acceptable salt of said a tautomer thereof or a pharmaceutically acceptable salt of compound or tautomer. said compound or tautomer. [0085] Yet another embodiment of the present invention is [0079] In a further embodiment of the compound of For the compound of Formula I in which R1 is hydrogen or ?uo mula Ia, R4 is independently ?uoro, chloro, methyl, ethyl, romethyl; R2 is methyl, ?uoromethyl, or l-methyl-lH-pyra propyl, methoxy, or ethoxy, Wherein said methyl, ethyl and zol-4-yl; and R3 is (2,2,2-tri?uoroethyl)aminomethyl or (1 -methoxypropan-2-yl)aminomethyl. propyl groups are optionally substituted With one to three ?uoro; and X is 0, l or 2; or a tautomer thereof or a pharma [0086] A further embodiment of the present invention is the compound of Formula I in which R1 is hydrogen or ?uorom ceutically acceptable salt of said compound or tautomer. In a ethyl; R2 is methyl, ?uoromethyl, or l-methyl-lH-pyrazol-4 further embodiment, R2 is methyl, optionally substituted With yl; R3 is (2,2,2-tri?uoroethyl)aminomethyl or (l-methox ?uoro; R4 is independently methoxy, chloro or ?uoro; X is 0, ypropan-2-yl)aminomethyl; R4 is ?uoro and X is l or 2. l or 2; or a tautomer thereof or a pharmaceutically acceptable [0087] Typically, a compound of the invention is adminis salt of said compound or tautomer. tered in an amount effective to treat a condition as described [0080] In a further embodiment of a compound of Formula herein. The compounds of the invention are administered by Ia, R2 is i(C(R5)2)m-(5-membered heteroaryl); R4 is inde any suitable route in the form of a pharmaceutical composi tion adapted to such a route, and in a dose effective for the pendently methoxy, chloro or ?uoro; m is 0; and X is 0, l or 2; treatment intended. Therapeutically effective doses of the or a tautomer thereof or a pharmaceutically acceptable salt of compounds required to treat the progress of the medical con said compound or tautomer. dition are readily ascertained by one of ordinary skill in the art [0081] In a further embodiment of a compound of Formula using preclinical and clinical approaches familiar to the Ia, R2 is i(C(R5)2)t4OR6; R6 is hydrogen, methyl or i(C medicinal arts. (R5)2)ni(C6_ 10 aryl), Wherein the aryl of R6 is phenyl option [0088] The compounds of the invention may be adminis tered orally. Oral administration may involve swalloWing, so ally substituted With substituents independently selected that the compound enters the gastrointestinal tract, or buccal from halogen, C1_6 alkyl, 4CH2F, 4CHF2, iCF3, iCN or or sublingual administration may be employed, by Which the iOH; R4 is independently methoxy, chloro or ?uoro; X is 0, compound enters the blood stream directly from the mouth. l or 2; n is l; and t is l; or a tautomer thereof or a pharma [0089] In another embodiment, the compounds of the ceutically acceptable salt of said compound or tautomer. invention may also be administered directly into the blood [0082] In a further embodiment of a compound of Formula stream, into muscle, or into an internal organ. Suitable means for parenteral administration include intravenous, intraarte Ia, R2 is i(C(R5)2)mi(C3_6 cycloalkyl), Wherein said rial, intraperitoneal, intrathecal, intraventricular, intraure cycloalkyl is optionally substituted With halogen or C 16 alkyl thral, intrasternal, intracranial, intramuscular and subcutane optionally substituted With one to three ?uoro; R4 is indepen ous. Suitable devices for parenteral administration include dently methoxy, chloro or ?uoro; m is 0 or 1; and X is 0, l or needle (including microneedle) injectors, needle-free injec 2; or a tautomer thereof or a pharmaceutically acceptable salt tors and infusion techniques. of said compound or tautomer. In a further embodiment, R2 is [0090] In another embodiment, the compounds of the cyclopropyl; and m is 0; or a tautomer thereof or a pharma invention may also be administered topically to the skin or ceutically acceptable salt of said compound or tautomer. mucosa, that is, derrnally or transdermally. In another US 2014/0323474 A1 Oct. 30, 2014 embodiment, the compounds of the invention can also be [0097] The compounds of the present invention may be administered intranasally or by inhalation. In another administered by any suitable route, preferably in the form of embodiment, the compounds of the invention may be admin a pharmaceutical composition adapted to such a route, and in istered rectally or vaginally. In another embodiment, the com a dose effective for the treatment intended. The active com pounds of the invention may also be administered directly to pounds and compositions, for example, may be administered the eye or ear. orally, rectally, parenterally, or topically. [0091] The dosage regimen for the compounds and/or com [0098] Oral administration of a solid dose form may be, for positions containing the compounds is based on a variety of example, presented in discrete units, such as hard or soft factors, including the type, age, weight, sex and medical capsules, pills, cachets, lozenges, or tablets, each containing condition of the patient; the severity of the condition; the a predetermined amount of at least one compound of the route of administration; and the activity of the particular present invention. In another embodiment, the oral adminis compound employed. Thus the dosage regimen may vary tration may be in a powder or granule form. In another widely. Dosage levels of the order from about 0.01 mg to embodiment, the oral dose form is sub-lingual, such as, for about 100 mg per kilogram of body weight per day are useful example, a lozenge. In such solid dosage forms, the com in the treatment of the above-indicated conditions. In one pounds of Formula I are ordinarily combined with one or embodiment, the total daily dose of a compound of the inven more adjuvants. Such capsules or tablets may contain a con tion (administered in single or divided doses) is typically trolled-release formulation. In the case of capsules, tablets, from about 0.01 to about 100 mg/kg. In another embodiment, and pills, the dosage forms also may comprise buffering the total daily dose of the compound of the invention is from agents or may be prepared with enteric coatings. about 0.1 to about 50 mg/kg, and in another embodiment, [0099] In another embodiment, oral administration may be from about 0.5 to about 30 mg/kg (i.e., mg compound of the in a liquid dose form. Liquid dosage forms for oral adminis invention per kg body weight). In one embodiment, dosing is tration include, for example, pharmaceutically acceptable from 0.01 to 10 mg/kg/day. In another embodiment, dosing is emulsions, solutions, suspensions, syrups, and elixirs con from 0.1 to 1.0 mg/kg/day. Dosage unit compositions may taining inert diluents commonly used in the art (e.g., water). contain such amounts or submultiples thereof to make up the Such compositions also may comprise adjuvants, such as daily dose. In many instances, the administration of the com wetting, emulsifying, suspending, ?avoring (e.g., sweeten pound will be repeated a plurality of times in a day (typically ing), and/or perfuming agents. no greater than 4 times). Multiple doses per day typically may [0100] In another embodiment, the present invention com be used to increase the total daily dose, if desired. prises a parenteral dose form. “Parenteral administration” [0092] For oral administration, the compositions may be includes, for example, subcutaneous injections, intravenous injections, intraperitoneal injections, intramuscular injec provided in the form of tablets containing from about 0.01 mg to about 500 mg of the active ingredient, or in another tions, intrastemal injections, and infusion. Injectable prepa embodiment, from about 1 mg to about 100 mg of active rations (e. g., sterile injectable aqueous or oleaginous suspen ingredient. Intravenously, doses may range from about 0.1 to sions) may be formulated according to the known art using suitable dispersing, wetting agents, and/or suspending about 10 mg/kg/minute during a constant rate infusion. agents. [0093] Suitable subjects according to the present invention include mammalian subjects. Mammals according to the [0101] In another embodiment, the present invention com prises a topical dose form. “Topical administration” includes, present invention include, but are not limited to, canine, feline, bovine, caprine, equine, ovine, porcine, rodents, lago for example, transdermal administration, such as via trans morphs, primates, and the like, and encompass mammals in dermal patches or iontophoresis devices, intraocular admin istration, or intranasal or inhalation administration. Compo utero. In one embodiment, humans are suitable subjects. sitions for topical administration also include, for example, Human subjects may be of either gender and at any stage of development. topical gels, sprays, ointments, and creams. A topical formu lation may include a compound that enhances absorption or [0094] In another embodiment, the invention comprises the penetration of the active ingredient through the skin or other use of one or more compounds of the invention for the prepa affected areas. When the compounds of this invention are ration of a medicament for the treatment of the conditions administered by a transdermal device, administration will be recited herein. accomplished using a patch either of the reservoir and porous [0095] For the treatment of the conditions referred to membrane type or of a solid matrix variety. Typical formula above, the compound of the invention can be administered as tions for this purpose include gels, hydrogels, lotions, solu compound per se. Alternatively, pharmaceutically acceptable tions, creams, ointments, dusting powders, dressings, foams, salts are suitable for medical applications because of their ?lms, skin patches, wafers, implants, sponges, ?bers, ban greater aqueous solubility relative to the parent compound. dages and microemulsions. Liposomes may also be used. [0096] In another embodiment, the present invention com Typical carriers include alcohol, water, mineral oil, liquid prises pharmaceutical compositions. Such pharmaceutical petrolatum, white petrolatum, glycerin, polyethylene glycol compositions comprise a compound of the invention pre and propylene glycol. Penetration enhancers may be incor sented with a pharmaceutically acceptable carrier. The carrier porated; see, for example, J. Pharm. Sci., 88(10), 955-958, by can be a solid, a liquid, or both, and may be formulated with Finnin and Morgan (October 1999). the compound as a unit-dose composition, for example, a [0102] Formulations suitable for topical administration to tablet, which can contain from 0.05% to 95% by weight of the the eye include, for example, eye drops wherein the com active compounds. A compound of the invention may be pound of this invention is dissolved or suspended in a suitable coupled with suitable polymers as targetable drug carriers. carrier. A typical formulation suitable for ocular or aural Other pharmacologically active substances can also be administration may be in the form of drops of a micronized present. suspension or solution in isotonic, pH-adjusted, sterile saline. US 2014/0323474 A1 Oct. 30, 2014 Other formulations suitable for ocular and aural administra [0108] The phrases “concurrent administration, co-ad tion include ointments, biodegradable (e.g., absorbable gel ministration,” “simultaneous administration,” and “adminis sponges, collagen) and non-biodegradable (e.g., silicone) tered simultaneously” mean that the compounds are admin implants, wafers, lenses and particulate or vesicular systems, istered in combination. such as niosomes or liposomes. A polymer such as cross [0109] The present invention includes the use of a combi linked polyacrylic acid, polyvinyl alcohol, hyaluronic acid, a nation of a BACE inhibitor compound as provided in Formula cellulosic polymer, for example, hydroxypropyl methyl cel I and one or more additional pharmaceutically active agent lulose, hydroxyethyl cellulose, or methyl cellulose, or a het (s). If a combination of active agents is administered, then eropolysaccharide polymer, for example, gelan gum, may be they may be administered sequentially or simultaneously, in incorporated together with a preservative, such as benzalko separate dosage forms or combined in a single dosage form. nium chloride. Such formulations may also be delivered by Accordingly, the present invention also includes pharmaceu iontophoresis. tical compositions comprising an amount of: (a) a ?rst agent comprising a compound of Formula I or a pharmaceutically [0103] For intranasal administration or administration by acceptable salt of the compound; (b) a second pharmaceuti inhalation, the active compounds of the invention are conve cally active agent; and (c) a pharmaceutically acceptable car niently delivered in the form of a solution or suspension from rier, vehicle or diluent. a pump spray container that is squeezed or pumped by the [0110] The compounds of this invention may also be used patient or as an aerosol spray presentation from a pressurized in conjunction with other pharmaceutical agents for the treat container or a nebulizer, with the use of a suitable propellant. ment of the diseases, conditions and/or disorders described Formulations suitable for intranasal administration are typi cally administered in the form of a dry powder (either alone, herein. Therefore, methods of treatment that include admin istering compounds of the present invention in combination as a mixture, for example, in a dry blend with lactose, or as a mixed component particle, for example, mixed with pho spho with other pharmaceutical agents are also provided. Suitable lipids, such as phosphatidylcholine) from a dry powder pharmaceutical agents that may be used in combination with the compounds of the present invention include, without limi inhaler or as an aerosol spray from a pressurized container, pump, spray, atomizer (preferably an atomizer using electro tation: [0111] (i) anti-obesity agents (including appetite suppres hydrodynamics to produce a ?ne mist), or nebulizer, with or without the use of a suitable propellant, such as 1,1,1,2 sants), including gut-selective MTP inhibitors (e.g., lomi tapide, usistapide, granotapide, dirlotapide, mitratapide, tetra?uoroethane or 1,1,1,2,3,3,3-hepta?uoropropane. For intranasal use, the powder may comprise a bioadhesive agent, implitapide and CAS No. 913541-47-6), CCKa agonists (e.g., N-benzyl-2-[4-(1H-indol-3 -ylmethyl)-5 -oxo-1-phe for example, chitosan or cyclodextrin. nyl-4,5-dihydro-2,3,6,10b-tetraaza-benzo[e]azulen-6-yl]-N [0104] In another embodiment, the present invention com isopropyl-acetamide, described in PCT Publication No. WO prises a rectal dose form. Such rectal dose form may be in the 2005/116034 or US Publication No. 2005-0267100 A1), form of, for example, a suppository. Cocoa butter is a tradi 5-HTZC agonists (e.g., lorcaserin), MCR4 agonists (e.g., com tional suppository base, but various alternatives may be used pounds described in US. Pat. No. 6,818,658), lipase inhibi as appropriate. tors (e. g., Cetilistat), PYY3_36 (as used herein “PYY3_36” [0105] Other carrier materials and modes of administration includes analogs, such as peglated PYY3_36, e.g., those known in the pharmaceutical art may also be used. Pharma described in US Publication 2006/0178501), opioid antago ceutical compositions of the invention may be prepared by nists (e. g., naltrexone), oleoyl-estrone (CAS No. 180003-17 any of the well-known techniques of pharmacy, such as effec 2), obinepitide (TM30338), pramlintide (Symlin®), tive formulation and administration procedures. The above tesofensine (NS2330), leptin, bromocriptine, orlistat, AOD considerations in regard to effective formulations and admin 9604 (CAS No. 221231-10-3) and sibutramine. istration procedures are well known in the art and are [0112] (ii) anti-diabetic agents, such as an acetyl-CoA car described in standard textbooks. Formulation of drugs is dis boxylase (ACC) inhibitor as described in WO2009144554, cussed in, for example, Hoover, John E., Remington’s Phar WO2003072197, WO2009144555 and WO2008065508, a maceutical Sciences, Mack Publishing Co., Easton, Pa., diacylglycerol O-acyltransferase 1 (DGAT-l) inhibitor, such 1975; Liberman et al., Eds., Pharmaceutical Dosage Forms, as those described in WOO9016462 or WO2010086820, Marcel Decker, New York, N.Y., 1980; and Kibbe et al., Eds., AZD7687 or LCQ908, a diacylglycerol O-acyltransferase 2 Handbook of Pharmaceutical Excipients (3rd Ed.), American (DGAT-2) inhibitor, a monoacylglycerol O-acyltransferase Pharmaceutical Association, Washington, 1999. inhibitor, a phosphodiesterase (PDE)-10 inhibitor, an AMPK activator, a sulfonylurea (e.g., acetohexamide, chlorpropam [0106] The compounds of the present invention can be ide, diabinese, glibenclamide, glipizide, glyburide, glime used, alone or in combination with other therapeutic agents, piride, gliclazide, glipentide, gliquidone, glisolamide, tolaza in the treatment of various conditions or disease states. The mide, and tolbutamide), a meglitinide, an (x-amylase inhibitor compound(s) of the present invention and other therapeutic (e.g., tendamistat, trestatin and AL-3688), an (x-glucoside agent(s) may be may be administered simultaneously (either hydrolase inhibitor (e.g., acarbose), an (x-glucosidase inhibi in the same dosage form or in separate dosage forms) or tor (e.g., adiposine, camiglibose, emiglitate, miglitol, vogli sequentially. bose, pradimicin-Q, and salbostatin), a PPAR y agonist (e. g., [0107] Two or more compounds may be administered balaglitazone, ciglitazone, darglitazone, englitazone, isagli simultaneously, concurrently or sequentially. Additionally, tazone, pioglitazone and rosiglitazone), a PPAR (x/y agonist simultaneous administration may be carried out by mixing (e.g., CLX-0940, GW-1536, GW-1929, GW-2433, KRP-297, the compounds prior to administration or by administering L-796449, LR-90, MK-0767 and SB-219994), a biguanide the compounds at the same point in time but at different (e.g., metforrnin), a glucagon-like peptide 1 (GLP-l) modu anatomic sites or using different routes of administration. lator such as an agonist (e.g., exendin-3 and exendin-4), lira
Description: