Haga clic acá PDF
Preview Haga clic acá
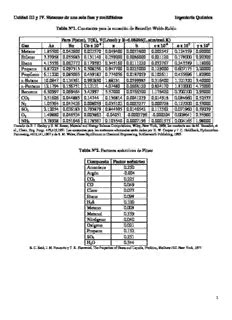
Unidad III y IV. Sistemas de una sola fase y multifásicos Ingeniería Química Tabla Nº1. Constantes para la ecuación de Benedict-Webb-Rubin Para P(atm), T(K), V(L/mol) y R=0.08206(L.atm/mol.K) Gas Ao Bo Co x 10-6 a b c x 10-6 α x 103 γ x 102 Metano 1.85500 0.042600 0.022570 0.049400 0.0033800 0.002545 0.124359 0.60000 Etileno 3.33958 0.055683 0.131140 0.259000 0.0086000 0.021120 0.178000 0.92300 Etano 4.15556 0.062772 0.179592 0.345160 0.0111220 0.032767 0.243389 1.18000 Propano 6.87225 0.097313 0.508256 0.947700 0.0225000 0.129000 0.607175 2.20000 Propileno 6.11220 0.085065 0.439182 0.774056 0.0187059 0.102611 0.455696 1.82900 n-Butano 10.0847 0.124361 0.992830 1.88231 0.0399983 0.316400 1.101320 3.40000 n-Pentano 12.1794 0.156751 2.12121 4.07480 0.0668120 0.824170 1.810000 4.75000 Benceno 6.50997 0.069464 3.42997 5.57000 0.0766300 1.176420 0.700100 2.93000 CO 2.51606 0.044885 0.14744 0.136814 0.0041239 0.014918 0.084660 0.52533 2 N 1.05364 0.047426 0.008059 0.035102 0.0023277 0.000728 0.127200 0.53000 2 SO 2.12054 0.026183 0.793879 0.844395 0.0146542 0.113362 0.071960 0.59239 2 O 1.49880 0.046524 0.003862 -0.04051 -0.0002796 -0.000204 0.008641 0.35900 2 NH 3.78928 0.051646 0.178567 0.103540 0.0007196 0.0001575 0.004165 1.98000 3 Tomado de E. J. Henley y E. M. Rosen, Material and Energy Balance Computations, Wiley, New York, 1969; las constants son de M. Benedict, et. al., Chem. Eng. Progr. 478,419,1951. Las constantes para las sustancias adicionales están dadas por H. W. Cooper y J. C. Goldfrank, Hydrocarbon Proccesing, 4612,141,1967 y de S. M. Wales, Phase Equilibrium in Chemical Engineering, Buttherworth Publishing, 1985. Tabla Nº2. Factores acéntricos de Pitzer Compuesto Factor acéntrico Amoniaco 0.250 Argón -0.004 CO 0.225 2 CO 0.049 Cloro 0.073 Etano 0.098 H S 0.100 2 Metano 0.008 Metanol 0.559 Nitrógeno 0.040 Oxigeno 0.021 Propano 0.152 SO 0.251 2 H O 0.344 2 R. C. Reid, J. M. Prausnitz y T. K. Sherwood, The Properties of Gases and Liquids, 3ºedition, McGraw Hill. New York, 1977. 1 Figura Nº1. Graficas de Cox para la presión de vapor (Tomado del A. S. Foust et. al., Principles of Unit Operations, Wiley, New York, 1960, p.550) 2 Figura Nº2. Carta de compresibilidad generalizada, presión reducida muy baja Tomado de D.M. Himmelblau, Basic Principles and Calculations in Chemical Engineering, 6ª edición.1997, p.285. 3 Figura Nº3. Carta de compresibilidad generalizada, baja presión. Tomado de D.M. Himmelblau, Basic Principles and Calculations in Chemical Engineering, 6ª edición.1997, p.286. 4 Figura Nº4. Carta de compresibilidad generalizada, para presión intermedias. Tomado de D.M. Himmelblau, Basic Principles and Calculations in Chemical Engineering, 6ª edición.1997, p.286. 5 Figura Nº5. Carta de compresibilidad generalizada, para presiones altas. Tomado de D.M. Himmelblau, Basic Principles and Calculations in Chemical Engineering, 6ª edición.1997, p.287. 6 Figura Nº6. Carta de compresibilidad generalizada, carta de Nelson y Obert. Tomado de D.M. Himmelblau, Basic Principles and Calculations in Chemical Engineering, 6ª edición.1997, p.287. 7 8 9 10
Description: