GENETIC RELATIONSHIPS AMONG FREMONTODENDRON (STERCULIACEAE) POPULATIONS OF THE CENTRAL SIERRA NEVADA FOOTHILLS OF CALIFORNIA PDF
Preview GENETIC RELATIONSHIPS AMONG FREMONTODENDRON (STERCULIACEAE) POPULATIONS OF THE CENTRAL SIERRA NEVADA FOOTHILLS OF CALIFORNIA
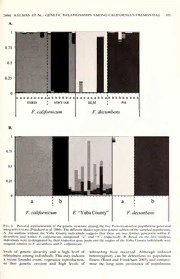
I Madrono, Vol. 53, No. 4, pp. 380-387, 2006 GENETIC RELATIONSHIPS AMONG FREMONTODENDRON (STERCULIACEAE) POPULATIONS OF THE CENTRAL SIERRA NEVADA FOOTHILLS OF CALIFORNIA Walter Kelman, Linda Broadhurst, and Curt Brubaker CSIRO Plant Industry, GPO Box 1600, Canberra, ACT2601, Australia email: [email protected] Albert Franklin Bureau of Land Management, Folsom Field Office, Folsom, California Abstract Fremontias, orflannelbushes{Fremontodendron), areadistinctiveelement ofCalifornia'schaparral communities. Fremontodendron decumbensisonly known from a fewpopulations in gabbro soil plant communities ofthe Sierra Nevada foothills in El Dorado County. Although a recovery plan forthese communities has been drafted, the long-term management of F. decumbens is complicated by its treatment as a subspecies of the more widespread F. californiciim, and by the recent discovery of additionalpopulationsofdecumbentplantsinYubaandNevadaCountiesthatarenoteasilyassigned to either F. californiciim or F. decumbens. Genetic relationships among 5 populations, including F. ca/ifornicum, F. decumbens, and the decumbent plants inYuba County, were ascertained usingAFLP markers. Principal coordinates and population structure analyses ofthe AFLP data showed that F. decumbensisgeneticallydistinguishablefromthepopulationsofF. ca/ifornicum that wesampled. This distinction, coupled with its unique morphology and ecology, support the treatment ofF. decumbens as a species and promote its continued conservation as a rare and unique element of plant communities on gabbro soils in the Sierra Nevada. The decumbent Yuba County population shared a numberofalleleswith F. ccdifornicum and F. decumbensand the analyses did not clearly distinguish its taxonomic relationships. It is possible that this population represents an historical hybrid between F. californicum and F. decumbens. A resolution of the taxonomic position of the decumbent Yuba CountypopulationswillrequiremorethoroughsamplingofF. ccdifornicum butthepresenceofunique alleles in this population suggests that it also should be conserved. Key Words: Fremontodendron, AFLPs, STRUCTURE, population genetics, conservation, gabbro soils. The flannel bushes or fremontias {Fremonto- themorewidespread F. californicum in theJepson dendron) are a distinctive element of chaparral Manual (Hickman 1993), the primary reference vegetation in the Sierra Nevada foothills and for the California flora; and secondly by the coastal ranges of Cahfornia, extending to scat- discovery of other decumbent populations of tered locations in central and western Arizona Fremontodendron that are not assignable to F. and the northern Baja California of Mexico. decumbens on morphological grounds. Kelman (1991) recognized three species: Fremon- Diagnostic features of F. decumbens are its | i todendron californicum (Torrey) Coville, F. mex- decumbent growth habit, orange to copper-red icanum Davidson, and F. decumbens R.M. Lloyd. flowers (as opposed to the yellow flowers of F. \ Fremontodendron californicum is the most wide- californicum and F. mexiccmum), dense stellate spread and polymorphic, while F. mexicanum is pubescence with long trichome rays on the | confined to southern California and the Baja abaxial leafsurface, and long peduncles. Kelman Californiapeninsula ofMexico. Fremontodendron (1991) noted the presence ofF. decumbens plants decumbens is an endemic element of the unique 1 km from the population at the summit ofPine gabbro soil plant communities of the Sierra Hill, and a large population of F. decumbens is i Nevada foothills, and was described from a pop- also present on the eastern boundary ofthe Pine ulation on the Pine Hill formation in El Dorado Hill Ecological Reserve (Boyd 2003). Beyond the j County (Lloyd 1965). It is known from onlya few Pine Hill site, decumbent plants ofFremontoden- populations at this site, and was listed as federally dron have been collected from a number of endangered in 1996 (USFWS 1996) and included localities in Yuba, Butte, and Nevada Counties. in a recovery plan for gabbro soil plants of the G. L. Stebbins collected plants from a population ! central Sierra Nevada foothills in 2002 (USFWS at a city dump site near Dobbins, Yuba County 2002). The long-term management of F. decum- in 1966. An occurrence of decumbent Fremonto- j bens as an endangered species is complicated by dendron, with "deeper orange and smaller and j two issues regarding its taxonomic rank: firstly by more campanulate" flowers was noted by Camp- |l| the treatment of F. decumbens as a subspecies of bell (1980) at a roadside locality near Campton- \ 2006] KELMAN ET AL.: GENETIC RELATIONSHIPS AMONG CALIFORNIA S FREMONTIAS 381 Table 1. Mean and Standard Error (in Brackets) for Diagnostic Morphological Characters of FremontodendronPopulations. ' Data from Kelman (1991), ' Measurements of 10 shrubs grown at Canberra, Australia, ^Basal branches well developed, plants as wide as tall. F. californicwii^ F. decumhens^ F. ''Yuba County"-^ n = 49 n = 48 n = 10 Habit erect decumbent^ decumbent Leaflength (mm) 42 (9) 32 (8) 27 (6) Leafwidth (mm) 25 (10) 25 (7) 27 (10) Trichome ray length (mm) 0.34 (0.19) 0.76 (0.14) 0.47 (0.10) Flower color yellow orange to mostly copper-red orange Flower diameter (mm) 46 (8) 41 (6) 26 (5) Peduncle length (mm) 9 (2) 17 (4) 6 (2) ville, Yuba County. In 1975, Lowell Ahart ccdifornicum, and the decumbent population near collected plants from a population at a dump Brownsville, Yuba County, and so provide site near Brownsville, Yuba County. Later a stronger basis for conservation decisions observations of this population indicated that affecting these taxa. although the plants were uniformly decumbent, the pale orange to yellowflowercolorand shorter Methods peduncles argued against their assignment to F. decumbens (Table 1). In Butte County, Ahart Leaf Sample Collection collected plants from a site on the Bloomer Road, east of Lake Oroville. This population was The locaHties ofthe five populations are shown described in the Manual of Vascular Plants of in Fig. 1. Fremontodendron decumbens leaf sam- Butte County as "low and stunted but apparently ples were collected from eight shrubs in the Pine not the ssp. decumbens (R.M. Lloyd) Munz ... Hill (PH) area ofEl Dorado County, all ofwhich known only from Eldorado and Nevada coun- were within 150 m of each other. A further nine ties". In 1985, a small population of decumbent plants were sampled from a nearby population plants was reported near Grass Valley in Nevada approximately 0.8 km north ofPine Hill (BLM), County (Marcia Braga personal communication). all within 20 m of each other. Fremontodendron Using the diagnostic characters, these plants were californicum leaves were collected from eight not assignable to F. decumbens in that flower plants on Tollhouse Road, Fresno County color was not as deep orange and the peduncles (THRD) and seven plants on Highway 168, and trichome rays were not as long as those in F. Fresno County (HWY168); the sites are approx- decumbensmeasured by Kelman (1991) (Table 1). imately 2.5 km apart. The "Yuba County" leaves Because these new decumbent populations are were sampled from nine shrubs from the popu- not assignable to either F. decumbens or F. lation near Brownsville, Yuba County (Yuba) californicum based on leaf length, trichome ray mentioned in the introduction. Four additional length and flower color, their taxonomic status samples were obtained from shrubs that were remains undetermined. grown in Canberra, Australia, from seed of A fundamental question concerning the con- the same Yuba County population, accessed servation management ofF. decumbens is wheth- as Commonwealth Plant Introductions (CPI er it represents a distinct taxon or is merely an 140372, CPI 140375, CPI 140376, and CPI ecotype of F. californicum, as implied by the 140378). Leaves were preserved using silica gel DNA transfer of F. decumbens to F. californicum by or lyophilized prior to extraction. Munz (1968). Likewise, the management of the newly identified decumbent populations depends DNA Extraction and AFLP Procedures on whether they are assigned to F. californicum, F. decumbens, or a new taxon. This problem was Total genomic DNA was extracted using emphasized in the recovery plan for Gabbro Soil 10 mg of dried leaf tissue ground to a fine Plants of the Central Sierra Nevada Foothills powder using 3 mm tungsten carbide beads in (USFWS 2002), which recommended that ''the a Retsch MM300 mixer mill and extracted using decumbent Fremontodendron within Nevada and the Qiagen 96-well DNEasy Extraction Kit Yuba Counties should be secured and protected (Qiagen, Melbourne). Amplified marker length unless they are determined not to be the listed [F. polymorphism (AFLP^"^) templates were pre- decumbens].'' Since morphological evidence has pared and selectively amplified using three AFLP not provided a clear answer to the species primer combinations £'-AGC:A/-CAT, E- ( placement of the decumbent plants in Yuba AGG:M-CTC, ^-AGG:M-CTG) following Bru- County, molecular markers were used to clarify baker and Brown (2003). The isccI-ANN primer the genetic relationships among F. decumbens, F. was end-labeled with [''P]-dATP and the AFLPs MADRONO 382 [Vol. 53 Population F. "YubaCounty" (39.46,-121.28) F.cdifornicum(HWY168) (37.052,-119.403) F.cdifornicum(THRD) (37.038,-119.39) O F.decumbens(BLM) (38.72,-120.988) F.decumbens(PH) (38.729,-120.98) Fig. 1. Location ofFreiuontodendron califbrnicuni, F. decumbens, and F. "Yuba County" populations sampled. were resolved on 6% denaturing polyacrylamide diversity (//e), the proportion of polymorphic gels run at 50watts using IX Tris-Taurine-EDTA loci (P), expressed as a percentage at the 5% (TTE) buffer (following Brubaker and Brown level as well as total gene diversity (H^) and 2003). The polyacrylamide gels were dried to the genetic diversity within populations (sites) (//§)• short plate and exposed to BiomaxMR (Kodak) Genetic differentiation among populations was film for one to four days. Autoradiograms were assessed using Wright's Fst following Lynch scored for the presence/absence of a total of 66 and Milligan (1994) with the confidence intervals polymorphic AFLP loci that were numbered (CI) estimated following 1000 permutations. sequentially from largest to smallest. Estimates of the pairwise relatedness coefficients between individuals (r) were calculated using Data Analyses AFLP-SURV following Lynch & Milligan's Taylor expansion (Lynch and Milligan 1994) and the Allele frequencies were calculated with aflp- relatedness matrix produced was used to extract SURVVersion 1.0(Vekemans 2002) usinga Bayes- and plot the first two principal coordinates ian approach that assumed Hardy-Weinberg (H- (PCO). Nei's (1978) genetic distance (D) was W) genotypic proportions with non-uniform calculated between population pairs using POP- prior distribution of allele frequencies (Zhivo- GENE (Yeh and Boyle 1997) and a UPGMA tovsky 1999) and used to estimate average dendrogram was constructed using ntsys 2.1Ix heterozygosity (Hj) which is analogous to gene (Rohlf2005). 2006] KELMAN ET AL.: GENETIC RELATIONSHIPS AMONG CALIFORNIA S FREMONTIAS 383 Table 2. Genetic Diversity in Fremontodendron Populations Assessed Using AFLPs. (n = number of individuals genotyped; P = percentage ofpolymorphic loci; //^ = Gene Diversity) Taxon Population n P Private Alleles BLM F. decwnheus PH 98 7225..29 00..122704 1 THRD F. californicwn HWY 8 74.1 0.228 2 7 63.0 0.222 F. "Yuba County" Yuba 13 72.2 0.284 5 Differentiation among populations and species The genetic differentiation between the F. was also assessed using STRUCTURE Version ccdifornicum and F. decumbens populations evi- 2.1, which is a model-based clustering method for dent in the principal coordinates analysis was inferring population structure (Pritchard et al. also apparent in the initial STRUCTURE 2000). In the first stage ofthe analyses, the two F. analysis. The most likely number ofinferred gene californicwn and the two F. decumbens popula- pools was four rather than two [mean In P(D) of tions were analyzed without using any prior —473 versus —625]. Both taxa were clearly information to determine the most likely number divided into two gene pools that were largely of sub-populations {K). Following this, individu- congruent with their population of origin als within each species were reassigned to their (Fig. 3A). When the origins ofthe Yuba County most likely sub-population and the origin of the individuals were inferred relative to this genetic "Yuba County" individuals was inferred relative background, it emerged as a genetically distinct to this genetic background. All analyses were element, a result that correlated with the high conducted using a 30,000 burn-in and 300,000 number of unique alleles present in this popula- runs and were replicated five times. tion (Table 2). Nonetheless, there were obvious genetic inputs to the Yuba County population Results from both F. ccdifornicum and F. decumbens (Fig. 3B), although the level of this influence Levels of genetic diversity varied among the varied substantially among individuals. The in- populations (Table 2). The F. decumbens BLM fluence ofF. ccdifornicum appeared to be greater population had the fewest polymorphic loci (P = than that ofF. decumbens, a result consistent with 22.2%) and lowestgenediversity {Hq, 0.120) ofall the principal coordinates analysis, where the the populations sampled. Levels among the other Yuba County individuals ordinated with F. populations were similar although the Yuba ccdifornicum along the first axis (Fig. 2A). A County population had the highest gene diversity structure analysis, in which no population in- (0.284), possibly reflecting the larger sample size formation was used, produced a congruent result ofthis population. Unique alleles were present in (i.e., there was unambiguous support for five PH (1), THRD (2) and Yuba (5). The low level of distinct populations). BLM genetic diversity in arose from the fact that there were only three multilocus genotypes in the Discussion population. In contrast, all individuals in the other three populations were genetically dissim- This study was prompted by uncertainty ilar. Population differentiation indicated that regarding the taxonomic rank of decumbent most of the total genetic diversity {H^ = 0.307) Fremontodendron populations and the conse- was apportioned within sites (//g = 0.226), with quences of this for their conservation manage- significant genetic differentiation among popula- ment. As evident in Table 1, a number of tions (FsT = 0.263, p < 0.001). diagnostic morphological features (in addition The first principal co-ordinate placed the two to habit) differentiate F. decumbens from F. F. decumbens populations in negative space while ccdifornicum. The genetic evidence presented here the F. ccdifornicum populations were in positive using measures of genetic distance (Fig. 2B) and space (Fig. 2A). Almost all the Yuba County population structure (Fig. 3A) also clearly sepa- individuals ordinated in positive space with the F. rates the two species. A taxonomic treatment that californicum individuals along the first principal merges these populations in a single species belies coordinate (Fig. 2A). As predicted by the F^t the genetic, morphological, and ecological dis- value, genetic distances among the five popula- crimination between the two taxa and weakens tions were also high and long branch lengths the case for the conservation ofthe F. decumbens separated F. ccdifornicum and F. decumbens in the populations of El Dorado County. UPGMA topology (Fig. 2B). The Yuba County Species with restricted distributions, such as F. individuals clustered with the F. californicum decumbens, generally exhibit lower levels of populations (Fig. 2B). genetic diversity than their more widespread MADRONO 384 [Vol. 53 A F. californicum -0.5 0.0 0.5 PCOl (13.7%) BLM YUBA Fig. 2. A. PrincipalcoordinatesanalysisofF. decumbens(P = PH, B = BLM), F. californicum(H = HWY168,T = THRD)andYubaCounty(Y)individualsbasedonLynchandMilligan's(1994)relatednessmatrix. B. UPGMA topology based on Nei's (1978) genetic distance (/)). counterparts (Hamrick and Godt 1989; Gitzen- genetically depauperate with respect to its wide- dannerand Soltis 2000), but this is not always the spreadcongener. However, furthersamplingofF. case and probably reflects the myriad of factors californicum is necessary to accurately quantify that can contribute to rarity (Karron 1987; the differences in genetic diversity between the Fiedler and Ahouse 1992). The F. decumbens two species. In contrast, plants of the other F. PH population had the highest levels of genetic decumbens population (BLM), which were within m diversity of the two species indicating it is not 20 of one another, had substantially lower 2006] KELMAN ET AL.: GENETIC RELATIONSHIPS AMONG CALIFORNIA'S FREMONTIAS 385 0.75 0.25 0.75 0.25 a b ; F. californicum Fig. 3. Pictorial representation ofthe genetic structure among the five Fremontodendron populations generated usingSTRUCTURE(Pritchardet al. 2000). Thedifferent shades representgeneticsubsetsofthesampledpopulations. A. An analysis without the Yuba County individuals suggests that there are two distinct genepools within F. decumhens and within F. ca/ifornicimu designated ''a" and "b", respectively. B. Based on the first analysis, individuals were re-designated by their respective gene pools and the origins ofthe Yuba County individuals were assigned relative to F. decumhens and F. californicum. levels of genetic diversity and a high level of inbreeding have occurred. Although reduced relatedness among individuals. This may indicate heterozygosity can be deleterious to population a recent founder event, vegetative reproduction, fitness (Reed and Frankham 2003) and compro- or that genetic erosion and high levels of mise the long term persistence of populations MADRONO 386 [Vol. 53 through poor seed set, smaller seed, slower indicates that this population should be con- germination response, poor seedling survival, served until further data are available. The and reduced reproductive capacity (Buza et al. morphological similarity and spatial proximity 2000; Oostermeijer 2000; Young et al. 2000; between the Yuba and Nevada County decum- Tomimatsu and Ohara 2003), this is not always bent populations predict they will be genetically the case, particularly for self-fertile species. While similar. When the single Nevada County in- further genetic and demographic investigation of dividual that we had access to was included in BLM the population is warranted, habitat loss is a separate principal coordinates analysis (not probably the most critical short-term process to presented), it fell within the Yuba County cluster, manage. but this population needs to be sampled more The taxonomic status of the more recently thoroughly before any robust conclusions can be discovered decumbent populations found in made. As noted above, there is a strong possibil- Yuba and Nevada Counties is more difficult to ity that the Yuba and Nevada County popula- resolve. They do not have the distinctive corre- tions originated from hybridization. The conser- lated characters of F. decurubeus and on other vation of hybrid populations is controversial morphological grounds would fall within the since introgression can lead to species extinction range of variation of F. californicwn, and if it (Allendorf et al. 2001). With increased fragmen- were not for their decumbent habit they would tation of natural habitats around the world, have been so treated. The genetic data for the however, once rare hybrid events may become Yuba County individuals is similarly ambiguous. more common place and could threaten species In the ordination analysis, the Yuba County integrity. The challenge for conservation man- individuals grouped within a larger cluster of agers is to determine whether hybridization F. californicufn individuals along the first axis, events are natural or anthropogenic (Allendorf and there were genetic inputs to the Yuba et al. 2001), and whether they pose a real threat. population from F. californicuni and F. decwu- Like their cogener, F. decunibens, the distribution heus. While a number of hypotheses could be of the other northern decumbent populations formulated to explain these relationships, the appears to be associated with gabbro or ultra- most likely scenario is that the Yuba County mafic substrates. It ishard to envision how one or population (and by inference the Nevada County more anthropogenic hybridization events would population) is derived from an historical hybrid- result in the apparent substrate-specific distribu- ization between F. californicuni and F. decunibens. tion observed. If the decumbent populations This could explain, in part, the higher levels of share adaptation to these unusual substrates, this genetic diversity observed in the Yuba county would be another indicator of a shared genetic population relative to F. ccdifornicum and F. heritage. Any proposed reintroduction of F. decunibens. Hybridization is a common event in decunibens should not use the Yuba and Nevada angiosperms and estimates of natural hybridiza- County populations as sources ofseed. Resolving tion among plant taxa range from 6 22% their taxonomic status will require a more worldwide (Ellstrand et al. 1996), primarily due extensive sampling of F. ccdifornicum popula- to the semi-permeable nature of plant reproduc- tions. tive barriers (Harrison 1993). Hybridization can While an understanding of the levels and lead to increased intra-specific genetic diversity, patterns of genetic diversity is an integral the origin and transfer ofgenetic adaptations, the component of species management, ecological origin of new ecotypes or species, and the and evolutionary interactions should also be reinforcement or breakdown of reproductive considered (Holsinger and Gottlieb 1991). Fre- barriers (see Rieseberg 1997). The unequal montodendron decunibens is pollinated by native contributions ofF. californicuni and F. decunibens bees (Boyd 1994), has seeds which are dis- to the Yuba County population could be persed by ants (Boyd 1996) and suffers high explained by subsequent backcrossing to the reproductive attrition from insects and rodents more widespread F. californicuni. That the genetic (Boyd 2003). Furthermore, F. decunibens and makeup ofthe Yuba County individuals can not the other decumbent populations are compo- be ascribed simply to F. californicuni and F. nents of plant communities that have varying decunibens suggests that the actual parental reestablishment strategies following fire (Marsh populations may not have been sampled (partic- and Ayres 2002). These mutualistic and com- ularly for F. californicuni), or that the hybrid munity relationships highlight the importance populations have existed long enough that back- of conserving these populations as integral ele- crossing and directional selection has substantial- ments of the Sierra Nevada gabbro flora (USFWS ly shifted gene frequencies. 2002). Our results do not support definitive conser- Acknowledgments vation recommendations regarding the Yuba and Nevada County populations, but the presence of We thank Marion Kilby and Janelle Scown for unique alleles in the Yuba County gene pool technical assistance. 2006] KELMAN ET AL.: GENETIC RELATIONSHIPS AMONG CALIFORNIA'S FREMONTIAS 387 Literature Cited Kelman, W. M. 1991. A revision o'i Frenwntodeudrcm AlleJs.netdKto.inrgWfe,cnonbFs.uerrWvg.a.,ti2Ro0.n0gFLu.iTdLheeehanprersy.o,bTlPre.emnsSdpwsriutienhlEhlyc,borlaiodngsdy: Lloy((dSSt,teerrRcc.uullMiia.accee1aa9ee6))5..fSArysotnmeemwtahtseipceSciBieeorstraaonfyNFer1ve6an:id3o-an2t0fo.odoetnhidlrlosn, California. Brittonia 17:382 384. BoydFa,rnedRm.oEivSio.tlo1ud9te9i4no.dnyPoo1ul6l:id6n1ea3ctui6mo2hn2e.bnisolo(Sgtyerocfultihaeceraaer)e.shMrau-b Lyncpho,pulMa.tiaonndgenBe.tiGc.stMriulctluirgeawni.th19R94A.PADnamlayrskiesrso.f Molecular Ecology 3:91-99. drono 41:277-289. Marsh, G. D. and D. R. Ayres. 2002. Genetic 1996. Ant-mediated seed dispersal of the rare . structure ofSenecio laynneae (Compositae): a rare chaparral shrub Fveinontodendron dccuinhcns (Ster- plant ofthe chaparral. Madrofio 49:150-157. culia2c0e0a3e.).FMacatdorrosfiaoffe4c3t:i2n9g9-s3e1e5d.production by the MuNZ, P. A. 1968. Supplement to a California ilora. end.angered chaparral shrub Froiioiitodcndroii ca/i- University ofCalifornia Press, Berkeley, CA. foruicum subsp. dccwnhens (Sterculiaceae). Madro- Nei, M. 1978. Estimation of average heterozygosity no 50:232-242. and genetic distance from a small number of Brubaker, C. L. anda. H. D. Brown. 2003. The use individuals. Genetics 89:583 590. ofmultiple alien chromosome addition aneuploids OOSTERMEIJER, J. G. B. 2000. Population viability facilitatesgeneticlinkagemappingofthe Gossipiimi analysis of the rare Geutiana pnciuuomuithe: the G genome. Genome 46:774-791. importance ofgenetics, demography and reproduc- BuzA, L., A. Young, and P. Thrall. 2000. Genetic tivebiology. Pp. 313-334///A. G. YoungandG. M. erosion, inbreeding and reduced fitness in frag- Clarke (eds.). Genetics, demography and viability mented populations of the endangered tetraploid of fragmented populations. Cambridge University pea Swainsoiui recta. Biological Conservation Press, Cambridge. 93:177-186. Pritchard, J. K., M. Stephens, and P. Donnelly. Campbell, D. 1980. A new location forFrenioutouden- 2000. Inference of population structure using dron decumhens in Yuba County. Four Seasons multilocus genotype data. Genetics 155:945-959. 6:14-15. Reed, D. H. and R. Frankham. 2003. Correlation Ellstrand, N. C, R. Whitkus, and L. H. Riese- between fitness and genetic diversity. Conservation berg. 1996. Distribution of spontaneous plant Biology 17:230-237. hybrids. Proceedings of the National Academy of Rieseberg, L. H. 1997. Hybrid originsofplant species. Science (USA) 93:5090-5093. Annual Review of Ecology and Systematics Fiedler, P. L. and J. J. Ahouse. 1992. Hierarchies of 28:359-389. cause: toward an understanding of rarity in Rohlf, F. J. 2005. NTSYSpc: Numerical taxonomy vascular plant species. Pp. 23^8 in P. L. Fiedler system. Exeter Publishing Ltd, Setauket, NY. and S. K. Jain (eds.). Conservation biology. The ToMiMATSU, H. AND M. Ohara. 2003. Genetic theory and practice ofnature conservation, preser- diversity and local population structure of frag- vation and management. Chapman and Hall, New mented populations of Tril/iuni canischatcense York, NY. (Trilliaceae). Biological Conservation 109:249-258. Gitzendanner, M. a. and P. S. Soltis. 2000. USFWS. 1996. Endangered and threatened wildlife and Patternsofgeneticvariation inrareandwidespread plants. Determination ofendangered status offour plant congeners. American Journal of Botany plants and threatened status ofone plant from the 87:783-792. central Sierran foothills of California. Federal Hamrick, J. L. AND M. J. W. GODT. 1989. Allozyme Register 61:54346-54348. diversity in plant species. Pp. 43 63 in A. H. D. USFWS. 2002. Recovery plan for gabbro soil plants of Brown, M. T. Clegg, A. L. Kahler, and B. S. Weir the Sierra Nevada Foothills. Region 1 U.S. Fish (eds.). Plant population genetics, breeding, and and Wildlife Service, Portland, OR. genetic resources. Sinauer Associates Inc., Sunder- Vekemans, X. 2002. AFLP-SURV version 1.0. Dis- land, MA. tributed by the author. Laboratoire de Genetique Harrison, R. G. 1993. Hybrids and hybrid zones: et Ecologie, Universite LibredeBruxelles, Belgium. historical perspective. Pp. 3-12 in R. G. Harrison Yeh, F. C. and T. J. B. Boyle. 1997. Population (ed.). Hybrid zones and theevolutionary processes. genetic analysis of codominant and dominant Oxford University Press, New York, NY. markers and quantitative traits. Belgian Journal Hickman, J. C. (ed.). 1993. TheJepsonmanual: higher of Botany 129:157. plantsofCalifornia. University ofCalifornia Press, Young, A. G., A. H. D. Brown, B. G. Murray, P. H. Berkeley, CA. Thrall, and C. Miller. 2000. Genetic erosion, HOLSINGER, K. E. and L. D. GOTTLIEB. 1991. Con- restricted mating and reduced viability in fragmen- servation ofrare and endangered plants: principles ted populations of the endangered grassland herb andprospects. Pp. 195-208 in D. A. Falk and K. E. Rutidosis Icptorrhyncoides. Pp. 335-359 in A. G. Holsinger (eds.). Genetics and conservation ofrare Young and G. M. Clarke (eds.). Genetics, de- plants. Oxford University Press, NY. mography and viability offragmented populations. Karron, J. D. 1987. A comparison oflevels ofgenetic Cambridge University Press, Cambridge. polymorphism and self-compatibility in geograph- Zhivotovsky, L. a. 1999. Estimating population ically restricted and widespread plant congeners. structure in diploids with multilocus dominant Evolutionary Ecology 1:47-58. markers. Molecular Ecology 8:907 913.
