Table Of ContentReference:Hiol Bull 193:47-61.(August. IW7)
Dose-Response Relationships for Experimental
Heterochrony in a Colonial Hydroid
NEIL W. BLACKSTONE
DepartmentofBiologicalSciences, NorthernIllinois L'niversiiv. DeKalh, IllinoisM)l15
Abstract. Hydractiniid hydroids display a range of and uncoupling again have similar effects, triggering
morphologicalvariation.Atoneendofthespectrum,the metabolicactivation(e.g., an increaseinoxygen uptake
coloniesgrowinasheet-likeconfigurationwiththeirpol- andashiftinthemitochondrialredoxstateinthedirec-
yps close together and short stolons. At the other ex- tion ofoxidation). In thecontextoftheoriessuggesting
treme,thecolonieshavearunner-likeforminwhichthe the metaboliccontrol ofdevelopment,adirecteffect of
polypsare fartherapart and connected by longstolons. feeding and uncoupling on colony development thus
These patterns exemplify the heterochronic variation cannotberuledout.Further,theremaybeaninteraction
found in many colonial animals and correspond to between flowrateand metabolism,sincegastrovascular
changes in the timingofthe production ofpolyps and flow distributes food throughout the colony, and since
stolon tipsrelativeto ratesofstolongrowthandcolony such substrate affects metabolic state. Both within-col-
maturation. Experimental studiesofclonal replicatesof onyflowrateand metabolism mayaffect heterochronic
a Podocoryne carnea colony demonstrated a dose-re- variationinthesehydroids.andmethodsappropriateto
sponse relationship between these heterochronic traits distinguishthesetwoeffectsarediscussed.
andwithin-colonygastrovascularflowtoperipheralsto-
lons. A dose-response relationship was found whether Introduction
flowwasperturbedbymanipulatingtheamountoffood
consumed by the colonies or by treating the colonies Evolutionary changes in the timing ofdevelopment
with2,4-dinitrophenol,anuncouplerofoxidativephos- underlie much ofanimal diversity (e.g.. Gould. 1977;
phorylation. In colonies in which flow was highly per- Alberch el a/.. 1979; Bonner. 1982; McKinney, 1988;
turbed by either treatment, a similar rate offlow pro- Wake el al.. 1991; Hall, 1992). Historically, studies of
ducedasimilarmorphologicalresponse.Thesedatasup- heterochrony have focused on describing patterns of
portthehypothesisofacausalrelationshipbetweenflow comparative embryology and morphology (Gould,
rateandheterochronicvariation.Nevertheless,flowwas 1977),andtoalargedegreethisdescriptiveandcorrela-
diminished by two clearly different mechanisms. Feed- tional tradition still persists(see Raft"and Wray, 1989).
ingmanipulation altered flow relativetothesizeofthe Thus, relatively little isknownabout theactual mecha-
stolon byalteringstolonthickness,withoutaffectingthe nismsthatgovernheterochronicvariation.Aremorpho-
absolutequantityofflow. Uncouplingwith dinitrophe- logical and life-history heterochronies direct conse-
noldiminishedtheabsolutequantityofflow,butdidnot quences ofgenetic and molecular heterochronies? Do
affect the size ofthe stolon. A plausible assumption is physiological,developmental,andmetabolicfactorsme-
that feeding manipulation affects the resistance ofthe diate heterochronic variation? Can unrelated genetic
stolontissuetoflow,orthefluidabsorptionofthistissue, changesproducesimilarheterochroniesbyaffectingthe
orboth; whereas uncoupling affectsthe amount ofen- same epigenetic process? Investigations ofsuch ques-
ergy available to drive the flow. At the level ofcellular tionsarecrucialifstudiesofheterochronyaretoprogress
metabolism, on the other hand, feeding manipulation beyond largely descriptive studiesofmorphology (Raff
andWray, 1989).
To address these sorts ofquestions, whole-organism
Received19September1996:accepted7May 1997. experimental manipulations have become increasingly
47
48 N. W. BLACKSTONE
common in studies of evolutionary morphology (cf. sexual reproduction) and paedomorphic (i.e.. "child-
KettersonandNolan, 1992;Sinervo >//., 1992;Sinervo shaped")adultmorphologies.
andBasalo, 1996).andthisisparticularlytrueinstudies Hydractiniidhydroidsillustratethesegeneralpatterns:
oftheevolutionofdevelopment(e.g..AlberchandGale. speciesofHydractiniaaretypicallysheet-like,whilespe-
1985: Stebbins and Basile, 1986; Meyer. 1987; Miiller. ciesofPodocorynearetypicallyrunner-like(Blackstone
1991; DeSalle and Carew, 1992; Blackstone and Buss. and Buss, 1991; Blackstone. 1996). The morphological
1992. 1993:Dudgeonand Buss. 1996).Thecentralmo- aspectsofthisvariation derivefrom the higherratesof
tivationfortheselatterstudiesisarticulatedbyStebbins polypandstolontipformation,relativetoratesofstolon
and Basile(1986) in theirdefinition ofphyletic pheno- growth and colony maturation, in Hydractinia com-
copies: "We propose this term for changes in form or paredtoPi>dncoryne. Thisdifferenceisparticularlypro-
physiological response that mimic the normal form or nouncedatthetimeoftheformationofthestolonalmat
reactionofarelatedphenotype,particularlyonebelong- in llydructiiiiii. In addition, the relative rates ofpolyp
ingtoadifferenttaxon. Usingthem, investigatorshave andstolonproductionshowan inversecorrelationwith
obtainedcluestothedevelopmental basisofevolution- ratesofgastrovascularfluidflowtoperipheralstolontips.
arychange,andoccasionallytothenatureandactionof ComparedtocoloniesofPodocorynecornea,maturecol-
the genes involved." Experimental aspects ofthis ap- onies of llYilnictiniu symbiolongicarpus exhibit a low
proach can be particularly effective in the context of rateofflowtoperipheralstolons,particularlysubsequent
studies ofheterochrony (e.g., Meyer, 1987). Neverthe- to the formation ofthe stolonal mat (Blackstone and
less,todefinitivelyinvestigatethebasisforheterochrony. Buss, 1992;Blackstone. 1996).
such experiments should include notjust gene dosage Experimental studies of heterochrony in these hy-
studies(e.g., DeSalleandCarew, 1992),butalsomanip- droidshavedemonstratedthatthebetween-speciespat-
ulations of physiological and developmental mecha- terncanbemimickedbyexperimental manipulationof
nisms against a uniform genetic background. Further, coloniesofasinglespecies(Blackstoneand Buss, 1992.
once an experimental basis for heterochrony has been 1993). Putatively,thesehydroidsincursubstantialener-
demonstrated, the causal basis of such a relationship geticcostsincirculatingthegastrovascularfluidthrough-
shouldbefurtherelucidatedbyestablishingtheunderly- outthecolony. Application of2,4-dinitrophenoltocol-
ingbiologicalgradientordose-responsecurve(e.g.. Hill, onies of Podocoryne carnea results in a condition of
1965;Weed, 1988). "loose-coupling" of oxidative phosphorylation. a de-
Asmodelsystemsforexperimentalstudiesoftheevo- creaseintheenergyavailableforgeneratinggastrovascu-
lution of development, clonal organisms (e.g., many larflow,andaconsequentdiminishingoftherateofflow
fungi,herbaceousplants,andcolonialinvertebrates)are to peripheral stolons. Correlated with this diminished
particularlyappropriate. In such organisms,thegrowth flowarechangesthat parallel patternsofheterochrony;
and development of the colony are inseparable, and therateofproductionofpolypsandstolontipsincreases
thereisabroadchronologicalwindowinwhichmanipu- relativetotheratesofstolongrowthandcolonymatura-
lations ofdevelopment are possible; moreover, geneti- tion, and peramorphic("shapesbeyond") forms result.
cally identical clonal replicatescan be used in theseex- Alternatively,gastrovascularflowcanbediminished by
periments(Bussand Blackstone, 1991). In manyclonal increasingthenumberoftimesacolonyisfed(e.g..from
groups, the morphologycan beidealizedascomprising 3to6timesperweek),possiblybecauseahigherrateof
feeding and reproductive entities, here termed polyps, feedingincreaseseithertheviscosityofthegastrovascu-
which are interconnected by vascularstolons. Runner- larfluid ortheresistanceto flow ofthestolonal tissues,
like forms(cf.. "guerrilla" ofHarper, 1985)showwidely orboth. Feedingmanipulation, liketreatmentwith un-
spacedpolypsandlongstolonalconnections,whilesheet- couplers, resultsinchangesthatparallelpatternsofhet-
like forms(cf. "phalanx" ofHarper, 1985)showclosely erochrony; again, the rate ofproduction ofpolypsand
packed polyps with short stolonal connections. These stolontipsincreasesrelativetoratesofstolongrowthand
differentmorphologicalpatternscorrespondtochangesin colonymaturation(seeBraverman, 1974).Althoughin-
thetimingoftheproductionofpolypsandstolontipsrel- creasedfeedingproducesasurfeitofnutrientsandseems
ativetotheratesofstolongrowthandcolonymaturation: in manywaystheoppositeoftheenergy-poorstatepro-
high rates ofproduction yield sheets, whereas low rates duced by uncoupling, its effect on colony physiology
yieldrunners.Further,thesedistinctivemorphologiescor- (i.e.. flow rate)issimilar. Incombination,thebetween-
relatewithavarietyoflife-historytraits;runner-likeforms species data (Blackstone and Buss, 1992; Blackstone,
tendtogrowquickly,reproduceearly,anddispersewidely 1996)and theexperimental manipulations(Blackstone
as compared to sheet-like forms (e.g., Jackson, 1979; andBuss, 1992, 1993)suggestthatflowrateisaprincipal
Harper, 1985). In theterminologyofheterochrony, run- mechanism underlying heterochronic alterations of
ner-like forms often exhibit progenesis (i.e.. precocious thesehydroidcolonies.
HETEROCHRONY DOSE-RESPONSE IN HVDRO1DS 49
Nevertheless,theseinterpretationsarecomplicatedby plications,andthemethodsappropriatefortheirresolu-
thesimilaritiespotentiallyinducedbyfeedingmanipula- tion, are discussed. At this time, it seems possible that
tion and uncoupling treatments at the level ofcellular both colony physiology(i.e.. flow rate)and metabolism
metabolism (e.g.. Chance et al., 1963; Heytler, 1981: maymediatethegeneticaspectsofheterochronyinthese
Chance. 1991).Briefly,feedingtriggersmetabolicactiva- hydroids.
tion, which has features (e.g.. increased oxygen con-
sumption, shift ofthe mitochondria! redox state in the MaterialsandMethods
directionofoxidation)thatcanbemimickedbyuncou-
Studyspecies
pling.Inthislattercase,oxidationofsubstrateis"uncou-
pled" from energy conversion, so metabolic activation Podocoryne carnca exhibits traits typical of runner-
does not lead to increased ATPformation. Treatments likecolonialanimals(Blackstone, 1996). Colonydevel-
involving feeding manipulation and uncoupling may opment begins with the metamorphosis ofthe planula
thussharesomecharacteristicsatthelevelofmitochon- larva into a primary polyp. Runner-like stolonsextend
drial metabolism, but diverge at the level ofATP pro- from the primary polyp. Stolons encase fluid-filled ca-
duction. Further,thefeaturesofmetabolicactivation in nalsthatarecontinuouswiththegastrovascularcavityof
experiments with uncouplers are often sensitive to the thepolyp.Incrosssection,stolonsconsistofafluid-filled
nature ofthe particular uncoupler used, its concentra- lumenencasedbyendoderm,ectoderm,andarigidperi-
tion,andotheraspectsoftheexperimentalprotocols. derm.Gastrovascularfluidcirculatesinthelumenofthe
Giventheseconsiderations,furtherinvestigationsinto stolonsandcarriesfoodand possibly other metabolites
the relationship between gastrovascular flow rate and fromthefeedingpolyptootherpartsofthecolony:con-
heterochronic variation in these hydroids are needed. tractionsofthemuscularpolyppropelthegastrovascular
Here, two series ofexperiments are reported. The first fluid (Schierwater et al., 1992). As the lumen fills and
seriesfocusedoncausalcriteria: ifadose-responserela- emptiesinresponsetocontractionsofthepolyps,theen-
tionshipexistsbetweenflowrateandheterochronicvari- dodermalandectodermaltissuelayersofthestolon ex-
ation,acausallinkbetweenthesevariablesgainsconsid- pandandcontractaswell.Therigidperiderm,however,
erablesupport(e.g..Hill, 1965:Weed, 1988).Hence,two remainsfixedandsetsthemaximumdiameterforstolo-
dose-responseexperimentswerecarriedoutwith clonal nalexpansion.Incrosssection,thestolon(andlumen)is
replicatesofthesameP. carncacolony.Thefirstexperi- notcylindrical but moreclosely resemblesa halfellipse
ment examined dose-response in feeding experiments. withthe"flat"surfaceadjacenttothesubstratum. Dur-
Althoughtheactualexperimentaltreatmentisthenum- ingtissueexpansionandcontractioninresponsetoflow,
beroffeedingsperweek,gastrovascularflowrate(thehy- thelumencrosssectionmaychangeshape,therebycom-
potheticalphysiologicaldose)isconsideredthepredictor plicating the physical biology offluid flow (see Black-
variable;morphologicaldevelopmentrelativetothetim- stone, 1996;VanWinkleandBlackstone, 1997).
ingofthesexual(medusoid)phaseofthelifecycleiscon- Colony development from a primary polyp can be
sideredtheresponsevariable.Thesecondexperimentex- mimicked by surgically explanting 1-2 polyps from a
amined dose-response in uncoupling experiments. colonyontoa newsurface. In both cases, P. carncade-
Again,gastrovascularflowrate(thehypotheticalphysio- velops by lineal extension ofthe stolons, initiation of
logical dose) is considered the predictor variable, and newstolonaltips,anditerationoffeedingpolypsonthe
morphologicaldevelopmentrelativetothetimingofthe stolons,andformsaloosenetworkofpolypsandstolons
sexual (medusoid) phase ofthe life cycle is considered typical ofmany runner-like forms. Once the available
theoutcomevariable. Bothexperimentssupportthehy- substratumiscovered,P.carneacoloniesincreasepolyp
pothesisofadose-responserelationshipbetweengastro- and stolonal tip formation, producing a more closely
vascular flow and heterochronic variation in these hy- knitnetworkofstolonsandultimatelyinitiatingthesex-
droids. ual(medusoid)phaseofthelifecycle. Whengenetically
Thesecondseriesofexperimentsdirectlyinvestigated identicalclonalexplantsofthesamecolonyareused,the
metaboliccharacteristicsofcoloniessubjectedtofeeding developmentoftreatedcoloniescanbemanipulatedrel-
manipulationanduncoupling;oxygenuptakeandmito- ative to control colonies, producing more sheet-like P.
chondria!redoxstateweremeasuredinclonalreplicates carnea and thus providing an experimental system to
ofthesameP. carneacolony. The resultssuggest some generally examine heterochronic variation in runners
parallelsbetweenthesetreatmentsatthelevelofcellular andsheets(Fig. 1).
metabolism,althoughtheextenttowhichsuch features
can directly affect colony development remains unex- Productionofcoloniesandcultureconditions
plored.However,theseresultscomplicatetheinterpreta- Coloniesonhermitcrabshellswerecollectedfromthe
tion ofthedose-response relationships, and thesecom- Yale Peabody Museum Field Station in Connectku .
50 N. W. BLACK.STONE
FigureI. Background-subtractedimagesofseveraltreatedandcontrolcoloniesofPodocorynecarnea:
(A)treatedwith 30nMdinitrophenol:(B)dinitrophenolcontrol;(C)fed6timesperweek;(D)feeding
control.Thegeneticallyidenticalcoloniesencrust 15-mmdiameterglasscoverslipsandwereimagedat
thetimeoftheinitiationofmedusaproduction(datafromtheseimagesareincludedinFigs.3and6).In
eachimage,polypsarebrightandcircular,stolonsaredarkerandweb-like,andthesubstratumappears
black.Bothincreasedfeedinganduncouplingincreasethenumbersofpolypsandencrustingstolonsatthis
developmentallandmark;suchcoloniescanbeconsideredperamorphic;/c,theyexhibit"shapesbeyond"
thenormaldevelopmentalpathways.
Clonal replicatesofoneP. carneacolonyweremadeby ulationexperiments,colonieswerefedtorepletionwith
explanting 1-2polypsandtheirconnectingstolonsonto brine shrimp nauplii 3days per week. Analysis has
round glass cover slips. For measures offlow rate and shown that, with similarculture conditions, "random"
morphology, 15-mm diametercoverslipsare ideal, but statistical effects (e.g.. time effects, tank effects, rack
formeasuresofoxygen uptakeand redox state. 12-mm effects;seeSokalandRohlf, 1981)arenegligible(Black-
diametercoverslipsarenecessary.Aftertheexplantsat- stoneandBuss, 1991).
tachedandbegantogrow,alltheoriginalexplanttissue
wasremoved from thecoverslip. Inallexperimentsex- Colonyimageanalysisandmorphometrics
cept the ones measuring redox state, colonies were
effectivelyconfinedtoonesideofthecoverslipsbycut- Coloniesweremeasuredusingimageanalysistechnol-
tingback encrusting stolons from the reverse side on a ogy(seeRohlfand Bookstein, 1990). Briefly,ahigh-res-
dailybasis.Coverslipsweresuspendedin floatingracks olution MTI CCD-72 cameraattached toa macro lens
andgrown in 120-literaquariacontainingReefCrystals wasusedtoprojectanimageofeachcolonyontoacolor
artificial seawater (salinity == 35%o) with temperature monitor interfaced with a PC compatible microcom-
controlto20.5 0.5C,undergravelfiltration,and50% puter(pentium/90MHz CPU, 32 Mb RAM)equipped
waterchanges weekly. Ammonia, nitrites, and nitrates withanOverlayFrameGrabberboard(640x480pixels
weremaintainedbelowdetectablelevels(AquariumSys- with 12-bit depth per pixel). OPTIMAS software was
temstestkits).Withtheexceptionofthefeedingmanip- usedtoacquirebackground-subtractedimagesofthecol-
HETEROCHRONV DOSE-RESPONSE IN HYDROIDS 51
onieswithilluminationappropriatetoproducethreedis- flowingseawaterwasadjustedwithathermoelectricde-
tinctluminancethresholds:thepolyps(lightest),thesto- vicetomaintainaconstantchambertemperature(20.5
lons(intermediate), and theemptycoverslip(darkest). 0.3C; chamber temperature was monitored with a
Usingthesethresholds,thesoftwareidentifiedandmea- YSI cuvette thermometer with a flexible probe). Colo-
suredtheareasoftheempty(i.e.,unencrusted)coverslip nieswereviewedonan inverted lightmicroscope(Zeiss
and of the individual polyps (Blackstone and Buss, Axiovert 135),witha40X Plan-Neoobjective in differ-
1992). Classification macros were used to identify and ential interference contrast. Three primary stolon tips
excludeareasofcoverslipoutsidetheedgeofthecolony. from each colony were videotaped with the MTI CCD
Thetotalcolonyareaandperimeterwerealsomeasured. camerafor 10minuteseach.
Data fileswereanalyzed using PC-SASsoftware. For Gastrovascular flow must be reversed in each distal,
eachcolonyateach measurementtime,thetotalareaof "dead-end"tip.Stolontipsfillasfluidenters;thevelocity
polyps and the total area ofempty, unencrusted cover ofthe fluid then decreases to zero (Fig. 2). Tips then
slipenclosedwithinthecolonywereexpressedasafrac- empty,andthefluid velocityagaindecreasestozero. In
tionofthetotal area(notethatthetotal areaofstolons theregionofthestolon immediatelybehindthetip,the
can be calculated as 1 minus this combined fraction). differencebetweenthewidthofthestolonlumenwhenit
Polyp area is clearly a measure ofpolyp development; isatamaximum(andfluidvelocityiszero)andwhenit
empty, unencrusted inner area is largely a measure of isata minimum (and velocity isagain zero)providesa
stolondevelopment. Althoughpolypscanshieldempty, measureoftherateofgastrovascularflow, ifthisdiffer-
unencrusted area from observation and measurement, ence is measured over time. These width or diameter
in practicethisisaminorsourceoferrorbecausestolon measuresare taken at the base ofthe lumen. With the
development is generally most extensive at the base of imageanalysissystemconnectedtotheVCR,thediam-
thepolyps.Thisisparticularlytrueatthetimeofinitia- eter of the stolon lumen was measured at a point
tionofthesexual(medusoid)phaseofthelifecycleand ~250/jm behindthetipitself. Inthisregion ofthesto-
subsequently. Thus, at the times in development when lon,gastrovascular fluid velocitygoesto zero asthelu-
morphology was measured (see below), polyparea and men diameterapproachesitsmaximum and minimum
unencrusted inner area behave as largely independent (thus velocity itselfneed not be measured). Lumen di-
measuresoftwodifferentaspectsofdevelopment. Mor- ameterwasmeasuredwhenthestolonwasfullandwhen
phologicaldifferencesbetweenexperimental treatments itwasempty,for3consecutive,butnonoverlapping,cy-
wereassessedbytwomethods:first,usingananalysisof cles.Foreachcycle,thenetlumenamplitude,thatis,the
variance with the outcome equal to the natural loga- differencebetweenthemaximumand minimumlumen
rithmoftheratiooftotalpolypareadividedbytotalun- diameters, was calculated. Periderm-to-periderm total
encrustedinnerarea:second,usingamultivariateanaly- stolonwidth(whichisinvariantthroughoutthecontrac-
sisofvariancewithtwooutcomes,thenaturallogarithm tioncycle)andtheperiod(inseconds)ofeachcyclewere
ofthe ratio oftotal polyp area divided by total colony alsomeasured(Fig.2).
areaandthenaturallogarithmoftheratiooftotalunen- Statistical analysis focused on the three measured
crustedinnerareadividedbytotalcolonyarea.(Natural outcomes: lumen amplitude, cycle period, and stolon
logarithmswereusedtobettermeettheassumptionsof width.Theinterpretationofthesemeasuresintermsof
parametric statistics.) Both analyses ask essentially the thevolumetricrateofgastrovascularflowhasbeendis-
samequestionsin slightly differentways, and bothgive cussed in detail elsewhere (Blackstone. 1996). Briefly,
very similarresults. To avoid redundancy, only the re- these measures can be combined into a biologically
sults ofthe MANOVA's are reported here. Additional meaningfulmeasureofgastrovascularflowrate:lumen
analyses usingother measuresofmorphology (e.g., the amplitude divided by cycle period and stolon width
ratiooftotal polypareato mean innerarea; see Black- (micrometersoflumendiameterpertotal micrometers
stone and Buss. 1992, 1993) provide very similar be- ofstolonwidthpersecond). Biologically,thisratemea-
tween-treatmenteffects. sureilluminatestherateofsupplyoffoodtothetissues
ofthe stolon tip. Both this rate measure and the indi-
I'ideomicroscopicmeasuresofperipheral ovifdpuaarlafmleotwripcarstaamteistteircss(gseeneerSaolklaylmaenedtRtohhelfa,ss1u9m8p1t).ioTnos
gastrovascularflow
comparetreatments, a nested analysisofvariancewas
Gastrovascular flow reaches a maximum 2-8h after used with cycles nested within stolons, stolons nested
feeding(Schierwaterelal., 1992);allofthesestudieswere withincolonies,andcoloniesnestedwithintreatments.
carriedout3-5 hafterfeeding.Thecolonywasplacedin In somecases, near-significanteffectsthatmaybebio-
aflow-through chamberwitha#1 coverslipasitsbase logically relevant are discussed (for justification, see
(Warner Instruments). The temperature of the in- Rothman, 1986).
52 N. W. BLACKSIONI
40- A;UTMMAENXWMIDUTMH epncrtiondretremrm
I
35- lumenwidth stolonwidth
30
lumencross-section
UQJ 25
20
15-
10
\LUMENWIDTH
ATMINIMUM
50 100 140 190 240
SECONDS
Figure2. Thecontractionandexpansionofthestolonlumenasittillsandemptieslargelyinresponse
ttoragnassvterrosveassceucltiaornHsowa,nd.Acmrpolsistsuedcetifoonrseoafcthhceysctloelcoonrtriepswpiotnhdsthteotlhuemdeinamaettmearxoifmtahlelaunmdenm.inSicmhaelmawtidatsh.hoIwn
theregionofthestolonimmediatelybehindthetip.thedifferencebetweenthewidthofthestolonlumen
whenitisatamaximum(andfluidvelocityiszero)andwhenitisataminimum(andvelocityisagain
zero)providesameasureoftherateofgastrovascularflow,ifthisdifferenceismeasuredovertime(modified
fromBlackstone
Feedingmanipulationexperiments formation (Blackstone and Buss. 1992. I993). Dinitro-
Eighteennewlyexplantedclonalreplicateson 15-mm cpohnetnaoilnitnrgea5t0memnltowfatshecaarprpireodproiuatteinsoglluatsisonp.etEriacdhischoels-
cover slips were randomly divided into three groups. onywaskeptinasinglepetridish. Disheswerearranged
Eachgroup received adifferent numberoffeedingsper on threetrays(one foreachtreatment) in an incubator
week (2X, 3X [control], and 6X). The morphology of (20.5C). To randomize position and shelfeffects, each
each colony was measured at the time the substratum colony'sdishwasshiftedbyoneposition eachday,and
becamecovered,whenmedusaproductionwasinitiated, trays were moved to a different shelfeach day. Petri
andwhenthefirst medusaewerereleased. Immediately disheswerechangeddaily.Whencolonieswerenotbeing
precedingthefirst measure,gastrovascularflowtothree treatedwithdinitrophenol,theywerekeptintheaquaria
stolon tips percolony was measured. The initiation of in the normal fashion. Colony morphologyand gastro-
medusaproductionwasdefinedasthedayafterthefirst vascularflow ratewere measured at the same develop-
medusa buds became visible on gonozooids ofthe col- mentallandmarksasinthefeedingmanipulationexper-
ony.Thetimeofthefirstreleaseofmedusaewasdefined iments.
as the day after maturing medusae (e.g.. tentacles ex-
tended and swimmingbell fully extended and contrac-
tile)werefirstobservedongonozooidsofthecolony. Dose-responserelationships
In evaluating dose-response relationships between
L'ncouplingexperiments physiologicalparameters(gastrovascularflowrateinthis
Eighteennewlyexplantedclonalreplicateson 15-mm case) and a morphological outcome, several considera-
cover slips were randomly divided into three groups. tionsarise. A considerabledifference isexpected in the
Eachgroupwastreatedwithadifferentconcentrationof rangeofvaluesbetweenaphysiologicaldoseandamor-
2. 4-dinitrophenol in seawater(0pM [control], \5nAl, phological response. A physiological measure is rela-
and30^Af)for4hperday.Suchtreatmentwithdinitro- tively instantaneous, whereasa morphological measure
phenol results in "loose-coupling" of oxidative phos- iscumulative. Hence, evenasmall physiological differ-
phorylation,oxidation ofNADH,anddiminishedATP encecan, overtime, producea largedifference in mor-
Ill II-ROCHRONY DOSE-RESPONSE IN HYDROIDS 53
phology. The same logicsuggeststhat thissort ofdose- ofseavvater (filtered to 0.2/jm). Chamber temperature
responserelationshipcanbestbeevaluatedbymeasures washeldconstant(20.5 0.02C)bymeansofanexter-
thatareoffsetintime.Inotherwords,themorphological nal circulationwaterbath(Neslab RTE-100D),andthe
effect of a physiological dose measured at one time rate ofdecline in oxygen concentration over a 30-min
shouldbeassessed by morphological measurestaken at period was measured (using a Strathkelvin 1302 elec-
latertimes.Giventheseconsiderations,themeanofnine trode and 781 oxygen meter) with stirring (by slowly
measures (from threecycles ofthree stolons) ofgastro- spinning the magnet, cover slips, and colony). Subse-
vascularflowtakenfromeachcolonyatthetimethesur- quently,thechamberwasopened.0.021 ml ofseawater
facebecamecoveredwasusedtopredict morphological removed, 0.021 ml of 1 mAIdinitrophenol solution in
outcomes taken subsequently (i.e., at the initiation of seawater added, the solution mixed and aerated thor-
medusaproductionandatthefirstreleaseofmedusae). oughly with a small pipette, and the chamber resealed
Dose-responserelationshipswereassessedgraphically (thisproceduretook ~7 min).Therateofdeclineinox-
andbyusingparametricandnonparametricmeasuresof ygen concentration was then measured for another
correlation.Gastrovascularflowrate(i.e.. lumenampli- 30min. Experiments were performed 3-5 h after the
tudedividedbycycleperiodandstolonwidth)wasused subject colony was fed, as part ofthe normal feeding
asthepredictor,andtheratiooftotal polypareatototal schedule. For the five colonies, the entire experiment
unencrusted inner area was used as the morphological thustooknearly2weeks.
outcome(theresultswerefoundtobe insensitivetothe Aftereachcolonywastested,acontrolexperimentwas
particular morphological metric used). Both measures carriedoutwith thesamefivecoloniesandan identical
werenaturallogtransformedtobettermeettheassump- protocol,exceptthatinsteadofaddinguncoupler.plain
tionsofthe parametric correlations. However, particu- seawaterwasadded priorto the second measure ofthe
larly in thecaseoftheoverfeedingexperiments, theas- declineinoxygenconcentration.Followingthesecontrol
sumption ofa bivariate normal distribution is notwell experiments,feedingexperimentsweredone,againwith
satisfied.Althoughall measuresofcorrelationweresim- thesame fivecolonieson the normal feedingschedule.
ilar,inthiscasethenonparametriccorrelationsaremost Inthiscase,asimilarprotocolwasused,exceptthatafter
appropriate, and only Spearman's Rsare reported here the first 30-min measure ofthedecline in oxygen con-
(Sokal and Rohlf. 1981). Correlations between individ- centration, thecolony wasremoved from thechamber,
ualflowparameters(lumenamplitude,cycleperiod,and fed a small amount ofbrine shrimp, carefully cleaned
stolonwidth)andthemorphologicaloutcomewerealso ofanyshrimporshrimpportionsremainingoutsidethe
tested. gastrovascular cavity, and returned to the chamber,
Finally,itshouldbenotedthatthedose-responserela- whichwasthenresealed(thisproceduretook ~20min).
tionships for the feeding manipulation and the uncou- Thedeclineinoxygenconcentrationwasthenmeasured
pling experiments may not be directly comparable. In again. Ineach oftheseexperiments,datawereanalyzed
particular, previousworkershaveshown thatconfining usingapaired-comparison/test.
colonies in petri dishes can lead to more sheet-like
growth(Miillerelat.. 1987; Plickert el a/.. 1987; Lange .Venture*ofmitochondrialredoxstale
and Miiller, 1991). In such confined cultures, soluble
morphogeneticfactorsproducedbythecoloniescanac- SpectrofluorometricassaysofNADVNADH provide
cumulateandenhancepolypandstolonproduction.The a useful measure of mitochondria! redox state (e.g.,
dinitrophenolexperimentsincludethiseffectinallthree Chance and Baltscheffsky, 1958; Chance and Thorell,
treatmentsand are thus not directly comparable to the 1959; Chance et til.. 1963; Chance. 1991). These mea-
entirelyaquarium-growncoloniesofthefeedingmanip- suresare carried out //; vivo, with no apparent damage
ulationexperiments. tothecolonies,usingaPerkin-Elmerspectrofluorometer
(excitation at 366nm and emission read near460nm)
Measuresofoxygenuptake andacryliccuvettes(whichdo notabsorboremitwhen
excitedabove300nm).Similarexperimentswithavari-
Fivenewlyexplantedclonalreplicateswereallowedto ety ofclonesofdifferent hydroid species(includingthe
grow on 12-mm cover slips for 2weeks. At that point, P. carnea clone used here) have already demonstrated
theeffectof30p.Mdinitrophenol on therateofoxygen that uncoupling with 30ftM dinitrophenol triggers a
consumptionofeachcolonywasmeasured.First,acover shift of the redox state in the direction of oxidation
slip,with itscolonyattached,wasaffixedwithadropof (BlackstoneandBuss, 1992, 1993;Blackstone,inpress).
siliconegreasetoacoverslipcementedtoasmall mag- Here, the effects offeeding were investigated. Eighteen
net.Thisassemblywascontained in a 13-mm diameter newly explanted clonal replicates were allowed to
sealedglasschamber(Strathkelvin RC300)with 0.7 ml completely cover both sides of 12-mm cover slips
54 N. W. BLACKSTONE
(~2 months).Toproduceanadequatesignal,threecol-
oniesoncoverslipswerereadsimultaneously.Thusthe
effectivesamplesizeis/;=6.Theemissionofeachgroup
wasmeasured;thecolonieswerethenremovedfromthe
cuvette, fed a small amount ofbrine shrimp, carefully
cleanedofanyshrimporshrimpportionsremainingout-
side the gastrovascular cavity, and returned to the cu-
vette forasecond measurement ofemission. A control
experiment was performed with the samecoloniesand
an identical protocol, exceptthatcolonieswere not fed
between emission readings. Data were analyzed using
paired-comparison/tests.
Results
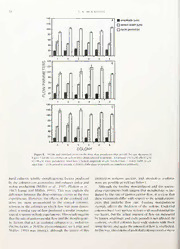
Feedingmanipulationexperiment*
RepresentativecolonyimagescanbeseeninFigure 1
andontheWorldWideWeb(http://www.bios.niu.edu/
eande/blackstone/blackstone.html). Feeding manipula-
tion substantially affects colony morphology such that
higher rates offeeding produce colonies with a greater
areaofpolypsand alesserunencrusted areawithin the
colony(Fig. 3).Atthetimetheycompletelycoveredthe
surface, coloniesin each treatment are roughly similar,
but there is a small between-treatment effect
(MANOVA, F = 2.93, df = 4, 28, P < 0.05). Subse-
quentlyincolonydevelopment,thedifferencesbetween
treatmentsbecome profound (Fig. 3; MANOVA at the
Piniti0a.ti0o0n1o;fMmAeNduOsVaAdevateltohpemfeinrstt.reFle=ase13o.f2.mdefdu=sa4,e,28F,
= 24.7, df= 4, 28, P 0.001). At thetimeofcovering
thesurface,thereisalsoasignificantbetween-treatment
differenceinflowrate(Fig.4;usingthecolonies-within-
treatmenteffectastheerrorterm,F= 8.9,df= 2, 15,P
<0.01)suchthatahigherrateoffeedingdiminishesthe
flow. Since flow rate is a composite ofthree measured
flow parameters (lumen amplitude, cycle period, and
stolonwidth),itisilluminatingtoexaminethebetween-
treatmentdifferencesinthesevariablesindividually.Lu-
men amplitude and cycle period show no significant
differences(Fig.5;F=0.20.df= 2, 15,P>0.8,andF=
1.43,df=2. 15. P> 0.25, respectivelyforeachvariable
using the colonies-within-treatment effect as the error
term). Stolon width, however, shows a dramatic be-
tween-treatmentdifference(Fig. 5:F= 23.8,df= 2, 15,
P <? 0.001, again using the colonies-within-treatment
effect as the error term). A higher rate offeeding pro-
ducesthickerstolons,andthebetween-treatmentdiffer-
ences in flow rate derive not from differences in the
amountofflow(amplitudeandperiod),butfromdiffer-
encesinthethicknessofthestolons.
Uncouplingexperiments
RepresentativecolonyimagescanbeseeninFig. 1 and
ontheWorldWideWeb(attheURLgivenintheprevi-
HETEROCHRONY DOSE-RESPONSE IN HYDROIIXS 55
5-
56 N. W. BLACKSTONF.
140' B
amplitude(um)
120: Dstolonwidth(um)
100: C] cycleperiod(s)
80-j
60:
40:
20- 23456
I I I I
*c
roo
oo
coo
oo
PARAMETERS
o
Sx*
FLOW
140-
120:
TOO:
60-j
40-j
20-