Division of Basic Sciences annual research directory PDF
Preview Division of Basic Sciences annual research directory
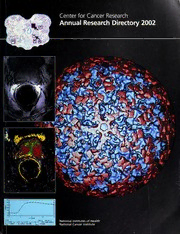
Center for Cancer Research Annual Research Directory 2002 1fn[m^«] 1.312 0.075 t - 1.165mfn CW_K0WP_M00ei) S<4.019)- 53.195k S_ - *7.2500 Cover: Center: Bovine papillomavirus fully bound by neutralizing monoclonal antibody #9 as seen in a 3D image reconstruction ofcryoelectron micrographs. The complex is viewed down a five-fold axis ofsymmetry. The density due to the antibody is shown in red and the density ofBPV1 is shown in blue. John Schiller, Laboratory of Cellular Oncology; and Benes Trus, Center for Information Technology Left, from top to bottom: 3D characterization ofthe prostate gland by coregistering whole-mountstep section MR histopathology; anatomic image; color-encoded Dynamic Contrast Enhanced MR! (DEMRI) depicting areas ofvascularperfusion andpermeability; andplot ofsignal intensity over time from prostate cancer. C. Norman Coleman and Cynthia Menard, Radiation Oncology Branch; Peter Choyke and Michael Knopp, Clinical Center/Diagnostic Radiology Department; reprintpermission for the whole-mountstep section histopathology obtained from Nature and from Cole KA, Krizman DB, and — Emmert-Buck MR. The genetics ofcancer a 3D model. Nature Genetics Supplement 1999;21:38-41. —— ————————————————————————— ———————— ——— — 1 Contents — 1 Office of the Director J. Carl Barrett, Ph.D. 9 Office of the Clinical Director Gregory Curt, M.D. Branches 1 Cancer Prevention Studies Branch Philip R. Taylor, M.D., Sc.D. 25 Cancer Therapeutics Branch Sandra M. Swain, M.D. 39 Cell and Cancer Biology Branch Kathleen Kelly, Ph.D. 65 Dermatology Branch Mark C. Udey M.D., Ph.D. 79 Experimental Immunology Branch Alfred Singer, M.D. 105 Experimental Transplantation and Immunology Branch Ronald E. Gress, M.D. 123 Genetics Branch IIan R. Kirsch, M.D. 143 HIV and AIDS Malignancy Branch Robert Yarchoan, M.D. 161 Medical Oncology Clinical Research Unit Ronald E. Gress, M.D. 175 Metabolism Branch Thomas A. Waldmann, M.D. 199 Neuro-Oncology Branch Howard Eine, M.D. 205 Pediatric Oncology Branch Lee J. Helman, M.D. 235 Radiation Biology Branch James B. Mitchell, Ph.D. — 245 Radiation Oncology Branch C. Norman Coleman, M.D. 259 Surgery Branch Steven A. Rosenberg, Ph.D., M.D. — 289 Urologic Oncology Branch 1/1/ Marston Linehan, M.D. Laboratories 297 Basic Research Laboratory-Bethesda Douglas R. Lowy M.D. 331 Basic Research Laboratory-Frederick Robert H. Wiltrout, Ph.D. 361 Laboratory of Biochemistry Claude Klee, M.D. 385 Laboratory of Biosystems and Cancer J. Carl Barrett, Ph.D. 395 Cancer and Developmental Biology Laboratory Colin L. Stewart, D.Phll. 403 Laboratory of Cell Biology Michael M. Gottesman, M.D. 421 Laboratory of Cell Regulation and Carcinogenesis Anita B. Roberts, Ph.D. 433 Laboratory of Cellular and Molecular Biology Lawrence E. Samelson, M.D. 443 Laboratory of Cellular Carcinogenesis and Tumor Promotion Stuart H. Yuspa, M.D. 461 Laboratory of Cellular Oncology Douglas R. Lowy, M.D. 477 Laboratory of Comparative Carcinogenesis Larry K. Keefer, Ph.D. 493 Laboratory of Experimental and Computational Biology Jacob V. Maizel, Ph.D. 515 Laboratory of Experimental Carcinogenesis Snorri S. Thorgeirsson, M.D., Ph.D. 525 Laboratory of Experimental Immunology John R. Ortaldo, Ph.D. 537 Gene Regulation and Chromosome Biology Laboratory Jeffrey N. Strathern, Ph.D. 553 Laboratory of Genetics Michael Potter, M.D. 563 Laboratory of Genomic Diversity Stephen J. O'Brien, Ph.D. 577 Laboratory of Human Carcinogenesis Curtis C. Harris, M.D. 585 Laboratory of Immune Cell Biology Jonathan D. Ashwell, M.D. 591 Laboratory of Immunobiology Berton Zbar, M.D. 599 Macromolecular Crystallography Laboratory Alexander Wlodawer, Ph.D. 611 Laboratory of Medicinal Chemistry Victor E. Marguez, Ph.D. 625 Laboratory of Metabolism PrankJ. Gonzalez, Ph.D. 637 Laboratory of Molecular Biology Ira Pastan, M.D. — ———— — — Laboratories (continued) 665 Laboratory of Molecular Cell Biology Carl Wu, Ph.D. — 669 Laboratory of Molecular Immunoregulation JoostJ. Oppenheim, M.D. 687 Laboratory of Molecular Pharmacology Yves Pommier, M.D., Ph.D. 701 Laboratory of Pathology Lance Liotta, M.D., Ph.D. 741 Laboratory of Population Genetics Kenneth Buetow, Ph.D. 751 Laboratory of Receptor Biology and Ge—ne Expression Gordon L. Hager, Ph.D. 765 Regulation of Cell Growth Laboratory Karen H. Vousden, Ph.D. — 779 Regulation of Protein Function Laboratory Allan Welssman, M.D. 785 Structural Biophysics Laboratory R. Andrew Byrd, Ph.D. 795 Laboratory of Tumor Immunology and Biology Jeffrey Schlom, Ph.D. Programs — 807 AIDS Vaccine Program Larry 0. Arthur, Ph.D. — 821 Computational Applications Program Stanley K. Burt, Ph.D. — 825 HIV Drug Resistance Program John M. Coffin, Ph.D. — 829 Resistance Mechanisms Laborato—ry Stuart F. J. Le Grice, Ph.D. 835 Retroviral Replication Laboratory Stephen H. Hughes, Ph.D. — 845 Molecular Targets Drug Discovery Program Michael Boyd, Ph.D. 847 Mouse Cancer Genetics Program—A/ea/ G. Copeland, Ph.D. Administrative Resource Centers 859 Building 10A 861 Building 10B 862 NCI-Frederick 863 Building 37 864 Building 41 865 IT Administrative Resource Center 867 Appendix A: Areas of Expertise by Investigator 871 Appendix B: NCI Faculties and Faculty Web Sites 873 Appendix C: Core Facilities and Resources 879 Appendix D: 2002 Intramural Research Award and Collaborative Project Award Recipients 881 Appendix E: Board of Scientific Counselors-A 883 Appendix F: Board of Scientific Counselors-B 885 Alphabetical Investigator Index 889 Keyword Index 901 Clinical Trials Index 1 Office of the Director Center for Cancer Research J. CarlBarrett, Ph.D. Directorofthe Center for Cancer Research, NCI Building 31 Room 3A1 Phone 301-496-4345 Fax 301-496-0775 E mail [email protected] Biography: Dr Barrettreceivedhis B.5. in chemistryat William and Mary College, Williamsburg, \/A, in 1969 and his Ph.D. in biophysical chemistryatJohns Hopkins University, Baltimore, MD, in 1974. Following a 3-yearpostdoctoral fellowship atJohns Hopkins University, Dr. Barrettbegan his careerat the NationalInstitute ofEnvironmental Health Sciences as leaderofthe Environmental Carcinogenesis Group, Laboratoryof PulmonaryPathoblology. In 1987, he became chiefofthe Laboratory ofMolecular Carcinogenesis where he conducted research on critical targetgenes In carcinogenesis molecularmechanisms ofenvironmentallyInduced cancers. His current research is centered on the relationship between aging and cancer, the genes involvedin cellularsenescence and differentiation, and the function ofKAI-1 and othercancermetastasissuppressorgenes. He is editor-in-chiefofMolecular Carcinogenesis, associate editorofCancer Research, anda memberofthe editorialboards of 12journals. He has been a chairperson, organizer, orkeynotespeakerat various conferences, workshops, andsymposia and has authored or coauthored over400publications. C. Norman Coleman, M.D. Deputy Director, CCR Building 10 Room B3B69 Phone 301-496-5457 Fax 301-480-5439 E mail [email protected] Biography: Dr. Coleman graduated from the University of Vermont with a B.A. In theoretical mathematics, then graduated from Yale UniversitySchool ofMedicine in 1970. He completed his Internship andresidencyIn Internalmedicine at the Universityof California In San Erancisco andat the NCI. Board-certifiedIn internal medicine, medical oncology, andradiation oncology. Dr. Coleman was a tenured facultymemberofthe Departments ofRadiologyand Medicine at the Stanford UniversitySchool ofMedicine beforejoining Harvard Medical Schoolin 1985asprofessorand chairman oftheJoint Center for Radiation Therapy. In 1999, he came to the NCIand became directorofthe new Radiation OncologySciences Program thathe created to coordinate radiation oncology activities within the NCI. He also serves the NCIas chiefofthe Radiation Oncology Branch, deputy directorofthe Center for Cancer Research, associate directorofthe Radiation Research Program, andspecialadvisor to the directorofthe NCI. He has written extensivelyin his fieldand has won numerous awards. 1 Lee Helman, M.D. J. Deputy Director, CCR Building 10 Room 13N240 Phone 301-496-4257 Fax 301-480-4318 E mail [email protected] Biography: Dr. Helman receivedhis M.D. magna cum laude in 1980 from the University ofMarylandSchool ofMedicine. He completed his clinical training In Internalmedicine at Barnes Hospital, Washington University, in 1983, andin medical oncologyat the NCI Pediatric OncologyBranch and Medicine Branch In 1986. He has heldpositions of increasing responsibilityat the NCIandbecame branch chiefIn 1997. Dr. Helman has a wide range ofresearch Interests andaccomplishmentsspanning moleculargenetics ofhuman cancerand development of new therapeuticapproaches topediatricsolid tumors. Douglas R. Lowy, M.D. Deputy Director, CCR Building 36 Room 1D32 Phone 301-496-4345 Fax 301-496-0775 E mail [email protected] Biography: Dr. Lowyreceivedhis M.D. from New York UniversitySchool ofMedicine In 1968. Between 1970 and 1973, he was a research associate In the LaboratoryofViral Diseases, NationalInstitute ofAllergyandInfectious Diseases, NIH. He trainedin Internal medicine atStanford Universityand dermatologyat Yale University, andstartedhis laboratoryat the NCI in 1975. He has been chiefofthe LaboratoryofCellular Oncology since 1983 anda deputydirectorsince 1996. He has received the Wallace RoweAward for Virus Research andhas been a memberofmanyscientificadvisoryboards, grants committees, and editorialboards. Robert H. Wiltrout, Ph.D. Deputy Director, CCR Building NCI-Frederick 428 Room 46 Phone 301-846-1258 Fax 301-846-6016 E mail [email protected] Biography: Dr. Robert Wiltroutobtainedhis Ph.D. in immunology from Wayne State University In 1979 andperformedpostdoctoralstudies on the regulation ofimmune responses with Dr. Ronald Herberman at the NCI. Hejoined the Laboratoryof ExperimentalImmunologyandbecame head ofthe Experimental Therapeutics Section in 1986. Dr. Wiltrouthas also servedas chiefofthe Basic Research Laboratorysince 1999. He has been a memberofmanyscientificadvisoryboards, extramural reviewgroups, and editorial boards. 2 — Stuart H. Yuspa, M.D. Deputy Director, CCR Building 37 Room 3B25 Phone 301-496-2162 Fax 301-496-8709 E mail [email protected] Biography: Dr. Yuspa received his B.5. fromJohns Hopkins Universityand his M.D. from the University ofMaryland MedicalSchool. He completed his internship and residencyat the Hospital ofthe UniversityofPennsylvania and has been a seniorinvestigatorat the NCIsince 1972. Among his honors are the Lila GruberAward oftheAmerican Academy ofDermatologyand the ClowesAward from theAmerican Association for Cancer Research. Dr. Yuspa is the authorofmore than 300publications In the fields ofcarcinogenesis and epithelial differentiation. Introduction The NCTs Center for Cancer Research (CCR) was created in March 2001 by merging two vital components ofthe NCTs Intramural Research Program the Division of Basic Sciences (DBS) and the Division of Clinical Sciences (DCS). This merger, initiated by former NCI Director Dr. Richard Klausner, is an important step in our goal to promote closer links between basic researchers and clinical investigators, thereby enhancing their opportunities for both scientific discovery and translational research (bench-to-bedside and bedside-to-bench). The CCRis also committed to supporting and training young scientists and clinicians as they launch their careers inbasic and clinical research. CCRoffers numerous predoctoral, postdoctoral, and clinical training positions with world-class scientists and physicians who are outstanding mentors and experts in their respective fields. The CCRis composed of over 300 principal investigators in 54 laboratories, branches, and programs. As one of the world's largest cancer research centers, the CCR takes advantage of the breadth of its researchers' training and experience to foster interdisciplinary programs and facilitate translational research. Basic research is a strength of the CCR, with areas of investigation including: immunology; carcinogenesis; human genetics; mouse genetics; DNA viral oncology; HIV; chromatinbiology; structuralbiology; replication and recombination; signal transduction; apoptosis; cell cycle regulation; cytokines and chemokines; cellular, molecular, and developmental biology; medicinal chemistry and natural products chemistry; molecular pharma- cology; xenobiotic metabolism; radiation biology; computational biology; and bioinformatics. Areas of excellence in clinical and translational research include: cancer vaccines, clinical proteomics, molecular targets of cancer, molecular imaging, biologic mediators, cell-based therapies, immunotoxin therapy, radiation therapy, cancer genetics, molecular epidemiology, cancer prevention, multidrug resistance, clinical pharmacology, angiogenesis, and molecular pathology. The CCRWeb site at http://ccr.nci.nih.gov offers detailed descriptions of thebasic research and clinical programs as well as links to current CCR clinical trials. The Newsletter and CCR Press section highlight some of the latest published findings from the Center. 3 New Scientific Opportunities in Interdisciplinary and Translational Research The CCR mission is to reduce the burden of cancer through exploration, discovery, and translation. The goals of the NCTs restructuring with the creation of the Center for Cancer Research are: • to foster interdisciplinary research • to facilitate translational research • to expedite technology development • to eiiliance training, particularly in interdisciplinary and translational research, and • to build partnerships between NCI and other NIH Institutes, federal agencies, academia, biotechnology companies, and the pharmaceutical industry The CCR along with the Division of Cancer Epidemiology and Genetics (the other NCI intramural division) has defined new institutional approaches to translate scientific knowledge towards achieving more effective cancer prevention, intervention, and treatment. The goal is to create an integrated, multidisciplinary research environment thatbrings together scientists from diverse fields to work on translating basic research findings into clinical applications. Collaboration, technology support and development, and access to resources are critical to achieving this goal. The NCI has responded to this challenge by establishing Faculties, which are composed of scientists from diverse laboratories and branches working cooperatively with a common interest in a particular discipline, disease, or approach to scientific discovery. Faculties foster collaboration, open access to new technologies and clinical resources, and challenge NCI researchers to become more involved in clinical research. Within the past year the following Faculties were established: • Molecular Targets • Immunology • Epidemiology, Carcinogenesis, and Prevention • Chemistry and Structural Biology • Cellular, Molecular, and Developmental Biology • Genetics, Genomics, and Proteomics • Breast Cancer • Gynecologic Oncology • Genitourinary Malignancies • HIV and Cancer Virology • Bioinformatics, Biostatistics, and Computational Biology Faculties provide a forum for scientists to engage one another to promote interaction and communication. Their goals are to promote translational and interdisciplinary research; to develop new technologies and resources; to enhance mentoring, recruitment, and training of fellows; to improve communication through retreats and seminars; to sponsor visiting scientists; to provide strategic planning and oversight; and to advise NCI leadership on important and innovative programs critical to the success of the NCI Intramural Program. Visit the Faculties Web site at http://ccr.nci.nih.gov for additional information and updates on their activities. The Medical Oncology Clinical Research Unit (MOCRU) was recently established to enhance the medical oncology clinical infrastructure and to enhance clinical investigation within the CCR. The MOCRU is a group of dedicated physicians who conduct clinical studies on a specific disease or MOCRU therapeutic area as a team effort. The is composed of Clinical Research Sections in breast cancer, genitourinary/gynecologic malignancies, vaccines, lymphoma, transplantation, immunotherapy, AIDS malignancies, lung/gastrointestinal cancer, and clinical genetics. Institutional support for the program is also provided through a Phase I Clinical Research Section, scientific core services, and offices for clinical operations, protocol support, research nurses, nurse practitioners, physicians' assistants, fellowship training, MOCRU translational research, and Navy-Oncology. The mission of the is to provide access to clinical research across the Center, excellence in clinical care, clinical training opportunities, and career growth for clinical staff. Technology Development and Support Technology development and support is yet another important goal of the CCR intramural program. Technology initiatives currently under development include clinical proteomics, molecular targets drug discovery, microarray technology, animal models development, and imaging tedmologies. The proteomics initiative involves the search for new serum markers for cancer; development of antibody chips, protein arrays, and reverse phase chips; a mass spectrometry center; a protein expression laboratory; and bioinformatics support. The molecular targets drug discovery program provides a full range of drug discovery scientific support: advising scientists on molecular target discovery, development of screening assays, conducting screens ofpure compound libraries, validation ofhits, and assistance in preclinical and clinical development ofpromising lead compounds. The microarray initiative uses modern lab automation and robotic methods for the production of gene microarrays to allow simultaneous study of the differential expression of large numbers of genes in normal, diseased, or treated cells. The animal models initiative includes transgenic and knockout core services, molecular and comparative pathology support, mouse proteomics, rodent imaging, phenotyping core support, and an animal brain tumor therapeutic and diagnostic core. The imaging initiative incorporates clinical imaging, advanced imaging applications, experimental and innovative technologies, and animal imaging into an interrelated imaging resources program. The CCRWeb site at http://ccr.nci.nih.gov features an initiatives section with additional details, contact information, and the latest projects. Mentoring and Training The CCR places a particular emphasis on training the next generation of investigators inbasic, interdisciplinary, and translational cancer research. Programs offered in the CCR include ACGME-accredited residency programs in anatomic pathology, radiation oncology, and dermatology. Additionally, ACGME clinical fellowship training programs in medical oncology, pediatric hematology/oncology, hematology/pathology, and cytology/pathology are available. Fellowships programs in surgical oncology, urological oncology, neuro-oncology, HIV and AIDS malignancy, gynecologic oncology, clinical cancer research for Ph.D.s, cancer epidemiology, cancer genetics, and cancer A prevention are also offered. Senior Clinical Research Fellowship is offered to outstanding clinical fellows to allow a period of intense training in translational research. The Center is involved in the Graduate Program Partnership initiative 5 tliat was recently established to attact graduate students to CCR laboratories. Areas of partnership currently under development include bioinformatics, chemistry, and comparative pathology. The Cancer Research Training Award (CRTA) and the Visiting Fellows (VF) program for foreign trainees are available in all the laboratories, branches, and programs. Visit the Web site for career and training opportunities. Partnerships with Academia and Industry The CCR is committed to forming partnerships that encourage technology development with industry, academia, and the private sector. CCR scientists and clinicians have a history of successful research collaborations with colleagues nationally and internationally. The CCR is also active in the area of technology transfer and strives to ensure that scientific breakthroughs reach the public through formal agreements between the government and industry. During the last year, there were over 100 active Collaborative Research and Development Agreements (CRADAs) between CCR investigators and outside institutions. For further information, contact the CCROffice of Research and Technology Partnerships at http://ccr.nci.nih.gov. Unique Aspects of the Intramural Research Program Thejuxtaposition ofbasic and clinical researchers in this large, diverse, yet highly interactive Center provides exceptional translational research and training opportunities. With the resources available at the NIHClinical Center, which houses over 50 percent of the NIH-funded general clinical research center beds in the United States, CCR scientists have a unique environment enabling them to move new drugs and diagnostics quickly from thebench to the bedside. Medical care is provided without charge to patients enrolled on NCI protocols. In addition, patients' travel is covered, and for children and minors participating in intramural studies, travel is also provided for a parent or guardian. One goal of the CCR is to capitalize on this extraordinary clinical resource. The CCR is a center of excellence for vaccine development and cell-based cancer immunotherapies utilizing specialized expertise, techniques, and facilities that exist within the Intramural Program. An example of the uniqueness of the Intramural Program is seen in the basic and clinical proteomics initiative, which is a collaboration between the NCI and the FDA. The program is built on laser capture microdissection technology, developed in the CCRLaboratory of Pathology, which involves identification and extraction ofmicroscopic homogenous cellular subpopulations from surrounding tissue. This technology is now being used to isolate tumor versus normal cellular subpopulations to identify potential molecular targets for cancer therapies. The long-range commitment needed to develop the technology to accurately identify specific targets for various cancers requires support that is unique to the Intramural Research Program. Another component ofthe proteomics initiative is the identification ofnovel markers for early cancer detection. These types of long-term, high-risk projects can accelerate the pace ofmedical research with public health importance and have an immeasurable impact on improving the nation's health care.
