Description of a new species of Eupsophus (Amphibia: Neobatrachia) from the Valdivian Coastal range, Southern Chile: an integrative taxonomic approach PDF
Preview Description of a new species of Eupsophus (Amphibia: Neobatrachia) from the Valdivian Coastal range, Southern Chile: an integrative taxonomic approach
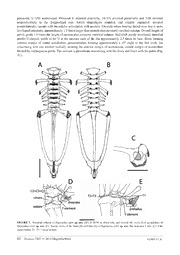
Zootaxa 3305: 53–68 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN1175-5334(online edition) Description of a new species of Eupsophus (Amphibia: Neobatrachia) from the Valdivian Coastal range, Southern Chile: an integrative taxonomic approach JOSÉ J. NUÑEZ1,3, FELIPE E. RABANAL2& J. RAMON FORMAS1 1Instituto de Ciencias Marinas y Limnológicas, Facultad de Ciencias, Universidad Austral de Chile. Casilla 567, Campus Isla Teja, Valdivia, Chile. 2Programa de Doctorado en Ciencias, Facultad de Ciencias, Universidad Austral de Chile. Casilla 567. Valdivia, Chile 3Corresponding author: E-mail: [email protected] Abstract We describe a new species of Eupsophus from the Coastal Range of Southern Chile. The new taxon can be differentiated using an integrative taxonomic approach that includes advertisement call, reproductive mode, tadpoles, and mtDNA D-loop sequences. Based on karyological characters, the new species is assigned to the roseus Group (2N=30), and differs from all described species therein by having early winter breeding season, terrestrial tadpoles and its advertisement call with spectral elements reaching the 20 kHz. Phylogenetic analyses based on mitochondrial data place the new species as sister taxon of E. migueli. The discovery of this new species highlights the importance of the multisource approaches to discover cryptic diversity as well as to establish the basis for prioritizing policies and conservation efforts on Chilean batrachofauna. Key words: Amphibia, Neobatrachia, Eupsophus altor sp. nov., integrative taxonomy, Chile Introduction The South American frogs of the genus Eupsophus Fitzinger 1843 are currently represented by ten species: E. emiliopugini Formas 1989, E. vertebralis Grandison 1961 (2n=28, vertebralis Group; Formas 1991), E.roseus (D & B 1841), E. calcaratus (Günther 1881), E. insularis (Philippi 1902), E. migueli Formas 1978, E.contulmoensis Ortiz, Ibarra-Vidal & Formas 1989, E. nahuelbutensis Ortiz & Ibarra-Vidal 1992, E. septentrionalis Ibarra-Vidal, Ortiz & Torres-Perez 2004 and E. queulensis Veloso, Celis-Diez, Guerrero, Méndez-Torres, Iturra-Constant & Simonetti 2005 (2n=30, roseus Group; Formas 1991) (Frost 2011). These species are restricted to the temperate Nothofagus forest of Southern Chile and Argentina (Formas 1979, Ibarra-Vidal et al. 2004). During a series of herpetological surveys (2003–2011) in the vicinity of Valdivia city (Cerro Oncol, 39°41’S; 73°18’W, Coastal range, Valdivia Province, Southern Chile; Fig. 1) we collected frogs that make their specific determination problematic because they have external morphological characters that overlap with those of two geographically close species: E. roseus and E. migueli. Preliminary comparative observations of the external morphology of the specimens from Cerro Oncol (Valdivia province) suggested that those specimens were much more similar to E. roseus than E. migueli and for that reason those specimens were temporarily named as Eupsophus aff. roseus. However, despite its external resemblance, the specimens from Cerro Oncol have different reproductive patterns when is compared with E. roseus. For example, the tadpoles of Eupsophus aff. roseus are fully developed in terrestrial environments (particularly under fallen trunks), while the tadpoles of E. roseus developed in small water-filled cavities in the ground near to streams (Formas & Vera 1980). In addition the advertisement calls Eupsophus aff. roseus were registered during the austral winter (May to August), while those of E. roseus are detected between October to January (Formas & Vera 1980). Integrative taxonomy is a conceptual multisource approach in which the nomination of a new species is considered as a hypothesis susceptible to be contrasted with different empirical evidences (Dayrat 2005, Padial et al. 2009, Schlick-Steiner et al. 2010). This proposition challenges subjective interpretations in the description and delimitation of new species, implicating that the resulting species hypothesis will be better supported as they are based on more comprehensive data from multiple sources. Accepted by M. Vences: 30 Mar. 2012; published: 9 May 2012 53 Taking into account these principles, and assuming that any kind of characters may be useful to propose species hypothesis, we proposed that the reproductive characteristics (reproductive season and terrestrial tadpoles) that present Eupsophus aff. roseus are enough to hypothesize that it belong to a independent lineage (sensu Baum & Donoghue 1995, De Queiroz 1998) of E. roseus and E. migueli and therefore constitutes a new species. To contrast our hypothesis we considered four lines of evidence: morphometry (nine continuous characters; see below), ethology (advertisement calls, reproductive mode) and molecular (mtDNA D-loop) data. FIGURE 1. Distributional map of Eupsophus altor sp. nov. Numbers indicate the following localities: 1 Cerro Oncol (Type Locality), 2 Curiñanco, 3 Chan-Chán, 4 Alepue. (See coordenates in the text). Material and Methods Sampling. Because the geographical distribution of the genus Eupsophus extends from the Central Chile (E. ′ ′ septentrionalis; 35º49S, Ibarra-Vidal et al. 2004) to the Southern Chile (E. calcaratus, 49º10S, Nuñez et al. 2011) the sampling area was reduced to the western slopes of the Coastal Range of the Valdivia province, between ′ ′ Mehuín (39º25S) and Cerro Oncol (39º41S), where our target taxon (Eupsophus aff. roseus) and other species of the roseus Group species occur (E. migueli and E. roseus). Morphometric characters. Morphological quantitative characters were analysed in 11 specimens of ′ ′ Eupsophus aff. roseus (6 males, 5 females; IZUA 3607-3618; Cerro Oncol; 39º41S; 73º19W, this paper), 10 ′ ′ specimens of E. roseus (3 males, 9 females, IZUA 3621-3633, La Saval; 39º48S; 73º15W; Nuñez et al. 1999) and ′ ′ 8 specimens of E. migueli (5 males, 3 females; IZUA 3634-3642; Colehual Alto; 39º22S;73º04W; this paper). The following variables were measured with a digital calliper (to the nearest 0.1 mm): snout-vent length (SVL), head length, head width, thigh length, tibia length, nostril-snout distance, nostril-eye distance, interocular distance and internarial distance. The Principal Component Analyses (PCAs) of morphometric characters was used to provide a representation of data useful to identify groups that may be related to previous assumptions about taxa without a 54 · Zootaxa 3305 © 2012 Magnolia Press NUÑEZET AL. priori subdivisions of the samples into discrete units (Wiley 1981). The PCA was performed with PAST v2.11 (PAleontological STatistics) (Hammer et al. 2001) on log10-transformed variables on variance-covariance matrices. Osteology. Osteological observations were made on two adult male specimens of Eupsophus aff. roseus (IZUA 3619-3620), cleared and double-stained skeletons using the methodology of Song & Parenti (1995). Tadpoles. Seventeen tadpoles of Eupsophus aff. roseus (IZUA 3643) stages 23–37 (Gosner 1960) were collected at Cerro Oncol. The following variables were measured with a digital calliper (to the nearest 0.1 mm): total length, body length, tail length, maximum tail height, tail muscle height, tail muscle width, internarial distance, and interocular distance. Chromosomal analysis. The chromosomes were studied on one female of Eupsophus aff. roseus (IZUA 3544) according the methodology described by Formas (1991). We analyzed five well spread c-metaphases plates. Centromeric positions were determined according to Levan et al. (1964). Call recording and analysis. During May and June 2009 the advertisement calls of five males of Eupsophus aff. roseus were recorded at Cerro Oncol with a digital recorder Marantz PMD 661 and an external unidirectional shotgun microphone (RØDE NTG2; frequency 20–20000 Hz). The spectral and temporal characteristics were analyzed using the software Audacity v1.2.6 at 44.1 kHz and 16 bit of resolution and a 512 FFT points. The Adobe Audition 3.0 was used to generate audiospectrograms and oscilograms. Calls were recorded at 30 cm from the emitter. Air and substrate temperature were measured immediately after each sound recording using a digital thermometer. The following quantitative parameters were analyzed: notes per call, call length (ms), fundamental and dominant frequencies (Hz), and maximum frequency (kHz). Molecular data. For DNA extraction, 12 specimens of Eupsophus aff. roseus were collected from Cerro ′ ′ Oncol (IZUA 3607–3618), 12 specimens of E.roseus from La Saval (IZUA 3497–3509; 39º48S; 73º15W), three ′ ′ specimens from Lago Pellaifa (IZUA 3510–3512; 39º37S; 71º59W), nine specimens from Bosque San Martín ′ ′ ′ (IZUA 3513–3521; 39º38S; 73º12W), six specimens of E. migueli from Mehuín (IZUA 3643–3648; 39º25S; ′ ′ ′ 73º12W), three specimens of E. calcaratus from Reserva Valdivia (IZUA 3526–3528; 40º10S; 73º29W), and one ′ ′ specimen of E. calcaratus from Bahía Mansa (IZUA 3529; 40°33S; 73°43W). Whole genomic DNA for PCR was extracted from blood or liver samples using standard protocols of chemical digestion (0.1M Tris–HCl pH 8.0, 0.2M EDTA, 1% SDS, 100 μg/ml proteinase K), followed by phenol/chloroform extraction (Sambrook et al. 1989). For amplification and sequencing of a fragment of the D-loop region, we used primers ControlP-H and ControlJ2-L (Goebel et al. 1999). Sequences were deposited in GenBank under Accession Numbers JQ780164–JQ780170. Nucleotide sequences were aligned using MAFFT v5 (Katoh et al. 2005) under the iterative method of global pairwise alignment (G-INS-i). Default settings were chosen for all the parameters involved. Phylogenetic trees were constructed by the DNA maximum-likelihood method implemented in the GARLI v0.951 program (Zwickl 2006) using parameters for molecular evolution model as suggested by jMODELTEST v1.01 (Posada 2008), under the Akaike Information Criterion (Posada & Crandall, 1998; Posada & Buckley 2004). We also used a Bayesian phylogeny estimation with MrBAYES v3.0 program (Ronquist & Huelsenbeck 2003). Each Markov chain was started from a random tree and run for 1.0x107 generations with every 1000th generation sampled from the chain. Stationarity was checked as suggested in Nylander et al. (2004). All sample points prior to reaching the plateau phase were discarded as ‘‘burn in’’ and the remaining trees combined to find the maximum a posteriori probability estimate of phylogeny. Results Morphometric data. The analysis of the morphometric characters of adult specimens based on Principal Component Analysis did not resolve discrete groups. In this analysis the Component 1 (eigenvalue = 24.06) explained 83.07% of the total variation and the Component 2 (eigenvalue = 1.98) explained 6.86%. Although the total variance explained is high (85.05%), the distribution of the individual values in the two factors did no conform discrete groups. A graphic representation of the eigenvalues of the first and second components is shown in Fig. 2. The measurements of males and females of Eupsophus aff. roseus,E.roseus and E. migueli are given in Table 1. A NEW SPECIES OF EUPSOPHUS FROM SOUTHERN CHILE Zootaxa 3305 © 2012 Magnolia Press · 55 FIGURE 2. Principal component analysis (PCA) of morphometric characters of Eupsophus aff. roseus, Eupsophus roseus and Eupsophus migueli. TABLE 1. Measurements (mm) of females and males of Eupsophus aff. roseus, E. roseus, and E. migueli. Means, standard deviation and ranges (parenthesis). Character Eupsophus aff. roseus E. roseus E. migueli females = 5, males = 6 females = 3, males = 9 females = 3, males = 5 Snout–vent length 38.2 ± 2.8 (33.5–42.0) 40.0 ± 3.2 (35.0–44.7) 39.4 ± 1.9 (31.2–47.2) Head length 17.7 ± 0.8 (11.6–14.3) 12.5 ± 1.4 (10.2–14.6) 12.8 ± 0.6 (10.2–15.1) Head width 14.2 ± 1.7 (10.9–15.1) 15.2 ± 0.9 (14.0–16.8) 14.4 ± 0.6 (11.9–16.9) Thigh lenth 18.9 ± 1.5 (15.7–20.6) 20.8 ± 1.9 (18.2–25.2) 20.4 ± 0.7 (17.5–23.0) Tibia length 19.5 ± 2.3 (12.9–21.0) 22.2 ± 1.4 (20.2–24.9) 20.9 ± 2.3 (17.8–24.1) Nostril–snout length 2.9 ± 0.5 (2.5–3.8) 3.6 ± 0.7 (1.9–4.6) 3.3 ± 0.1 (2.7–3.8) Nostril–eye distance 3.0 ± 0.5 (1.9–3.8) 3.5 ± 0.6 (2.6–4.7) 3.6 ± 0.2 (2.9–4.5) Interocular distance 5.5 ± 0.6 (4.6–6.4) 6.1 ± 0.7 (5.0–7.5) 5.7 ± 0.3 (4.8–7.5) Internarial distance 3.9 ± 0.3 (3.4–4.3) 4.0 ± 0.4 (3.5–4.5) 4.1 ± 0.1 (3.6–4.6) Advertisement calls. The advertisements call of Eupsophus aff. roseus (air temperature 10.0–12.5°C) consists of a single note with the following characteristics: note duration 290–360 (336 ± ms), dominant frequency ranges between 1317–2098 (1882 ± 250 Hz) and the fundamental frequency ranges 304–1298 (756 ± 187 Hz). These vocalizations are rich in harmonics and show spectral elements which reach 20 kHz (Fig. 3). With the same method used to record the advertisement call of Eupsophus aff. roseus, one of us (FER) recorded the vocalizations of E. migueli and E. roseus. His results (not included herein) were in agreement to those previously obtained by Formas (1985) to E. migueli and Márquez et al. (2005) to E. roseus (Table 2). 56 · Zootaxa 3305 © 2012 Magnolia Press NUÑEZET AL. TABLE 2. Advertisement calls characteristics (means and ranges) of Eupsophus aff. roseus, Eupsophus roseus and Eupsophus migueli. (n = number of males recorded). Species Notes per call Call length (ms) Fundamental Dominant Maximum References frequency (Hz) frequency (Hz) frequency (kHz) E. aff. roseus 1 336 756 1882 > 20 This paper n = 15 (290–490) (1317–2098) (1317–2098) E. roseus 1 258 632 1871 < 15¹ Marquez et al. n = 7 (124–235) (346–1018) (1503–2166) 2005 E.migueli 1 240 450 1835 < 15¹ Formas 1985 n = 12 (200–350) (390–987) (1500–2500) ¹ F. Rabanal; pers.obs. FIGURE 3. Sonogram (A), oscillogram (B) and power spectrum (C) of the advertisement call of Eupsophus aff. roseus (air tempature 10.0–12.5°C). Bar indicates 1 ms. Molecular relationships. Hierarchical likelihood test implemented in jModeltest (Posada 2008) selected a Γ TRN+I+ substitution model as best fitting the data (A = 0.2917, C = 0.2279, G = 0.1373 and T = 0.3431; gamma shape parameter = 0.1480). The single phylogram obtained from Maximum Likelihood analysis (Likelihood score -lnL =1200.5051) is shown in Fig. 4. Bayesian searches resulted in a topology that completely agreed with Maximum Likelihood analysis. This analysis shows two results: first the specimens assigned to Eupsophus aff. roseus are clearly differentiated from E. roseus and second that Eupsophus aff. roseus share in this analysis a common ancestor with E. migueli. Taxonomic Conclusions. Two independent lines of evidence (bioacoustics and molecular) allow to us to conclude that Eupsophus aff. roseus is a new species. This conclusion, together our initial observations (winter breeding season and terrestrial oviposition/clutches and tadpoles) supports the hypothesis that Eupsophus aff. roseus belongs to a lineage distinct from E.roseus and E. migueli. A NEW SPECIES OF EUPSOPHUS FROM SOUTHERN CHILE Zootaxa 3305 © 2012 Magnolia Press · 57 FIGURE 4. Single tree recovered in the Maximum Likelihood analysis; Bayesian analyses recovered a consensus tree with identical topology. The support indexes for each of the nodes are Maximum Likelihood and Bayesian posterior probabilities. Labels correspond to species and sampling localities (RV =Reserva Valdivia, LP =Lago Pellaifa, SM = Bosque San Martín, SV =La Saval, BM = Bahía Mansa). Eupsophus altor sp. nov. Nuñez, Rabanal & Formas (Fig. 5) Oncol’s ground frog (English) Rana de hojarasca de Oncol (Spanish) Type Material. Holotype. IZUA 3607, adult male collected by Felipe Rabanal and José Nuñez on 11 June 2008 at the Cerro Oncol (39º41’S; 73º19’W, 650 m a.s.l.), Coastal range, Valdivia Province, 40 km W (by road) of Valdivia city, Chile (Fig. 1). Paratypes. IZUA 3608–3620, collected at the type locality, same data as holotype. 58 · Zootaxa 3305 © 2012 Magnolia Press NUÑEZET AL. Diagnosis. The species is assigned to the genus Eupsophus because it has the following osteological cranial pattern (cervical cotylar arrangement type II; palatal shelf of premaxilla relatively deep; palatal shelf of maxilla of moderate width; pterygoid process moderately large; nasals small, widely separated medially; nasals in broad contact with maxillae, not in contact with pterygoids; epiotic eminences prominent; zygomatic ramus of squamosal of moderate length, widely separated from maxilla; otic ramus of squamosal as long as zygomatic ramus, expanded medially into small otic plate; squamosal-maxillary angle 50–55°; palatines broad, widely separated medially, bearing odontoid ridges; sphenethmoid entire, extending anteriorly to anterior edge of nasals parasphenoid alae oriented at right angles to anterior ramus of parasphenoid, broadly overlapped laterally by median rami of pterygoid) as has been described by Lynch (1971) and endotrophic tadpoles (Formas 1985). Eupsophus altor is assigned to the E. roseus Group species by having 2n = 30 chromosomes. Eupsophus altor differs from all species described of the E. roseus group species by having early winter breeding season, terrestrial tadpoles and its advertisement call with spectral elements reaching the 20 kHz, and nine mitochondrial D-loop nucleotide site substitutions from its congeneric species phylogenetically closest. FIGURE 5. A. Holotype of Eupsophus altor sp. nov. (IZUA 3607) from Cerro Oncol (Valdivia Province). B: Terrestrial embryos of Eupsophus altor sp. nov. C: Nest with tadpoles of Eupsophus altor sp. nov. Stage 30. D: Tadpoles at stages 37–41. Stages according to Gosner (1960). Bar indicates 10 mm. Description of the holotype. Adult male 36.6 mm in SVL. Head 0.93 times narrow than body; head length 31.7% of SVL; head width 1.2 times broader than long. Snout rounded dorsal view and obtuse in lateral view; loreal region flat, nostrils slightly prominent, oriented laterally; internarial distance 0.31 times the head width, internarial region slightly convex; nostril slightly closer to the anterior border of the eye than the terminus of snout; canthus rostralis marked. Eyes prominent, laterally oriented, 0.44 times the head length; tympanum round, 0.67 times the eye diameter; dorsolateral fold well developed, extending from the posterior corner of eyelid, terminating dorsal to forelimb. Maxillary and premaxillary teeth present; seven prevomerine teeth (4 on the left and 3 on the right), obliquely located between the choanae, small sized (0.26 times internarial distance) and subcircular in shape; tongue rounded, posterior the border slightly notched, attached approximately 66.4% of its length interiorly. Forelimbs slender; dorsal and ventral surfaces smooth. Relative length of the fingers: III>IV>II>I; tips of the І ІІ І fingers rounded and slightly protuberant; subarticular tubercles rounded distributed on fingers as follows: - - V A NEW SPECIES OF EUPSOPHUS FROM SOUTHERN CHILE Zootaxa 3305 © 2012 Magnolia Press · 59 ІІІ (1), (2); inner palmar tubercle ovoid; outer almost rectangular, as long as the inner; one subarticular tubercle on fingers I–IV; supernumerary palmar tubercles absent; nuptial pads on fingers I–II with tiny spines unpigmented. Hind limbs long and slender (162.8% of SVL); tibiotarsal articulation reaching the posterior border of eye when hind limb is adpressed along the body; toes long and thin, their relative length are: IV>V=III>II>I; a small delicate web between toes III–IV; tips rounded; inner metatarsal tubercle ovoid and developed, external tubercle conical and small, one-fourth of length of inner metatarsal tubercle; subarticular tubercles rounded, distributed on toes as follows: I(1), II(1), III(2), IV(3), V(2). Skin of head, dorsal surface, flanks and limbs smooth; ventral surfaces of limbs smooth; cloacal opening directed posteriorly at ventral level of thighs; cloacal opening unornamentated, covered by a fold of skin; Ventral surface of thighs smooth. Colour in life. The dorsum is reddish-pink with light gray spots. Extremities with light gray bracelets. The flanks are whitish but with yellow in axillary and inguinal areas. Throat, chest and belly with minute melanophores regularly distributed. The ventral surface is white or creamy (Fig. 5). Upper part of the iris yellowish with black reticulations. Color in preservative (70% ethanol plus NaCl) similar to the live specimens. Osteology. Neurocranial braincase: The neurocranial braincase is made up of three bones: sphenethmoid, prootics and exoccipitals. It is partially covered by the frontoparietals dorsally and the parasphenoid ventrally (Fig. 6A). The sphenethmoid forms the floor, edges of the roof and the anterolateral wall of each side of the braincase. Dorsally occupies a large area (its width is equal to the length of the nasals) between the nasals, frontoparietals and frontoparietal fontanella. Its ventral face overlaps with the inner half of the neopalatines, vomers and anterior end of the cultriform process of the parasphenoid. The prootics are fused with the exoccipitals, forming the posterior region of the braincase and the otic capsules. The exoccipital, which are covered ventrally by the parasphenoid forming the posteromedial walls of the otic capsules, the margin of the foramen magnum, and the occipital condyles. Dermatocranium: The frontoparietals are paired and their anterior extremes are slightly divergent. The frontoparietals are expanded posteriorly and they overlap the prootics but do not reach the foramen magnum. The frontoparietal fenestra extends forward from the anterior third of the skull. The fontanella is 0.24 times the skull length. The nasals are paired subrectangular bones, transversally oriented and superimposed on the sphenethmoid (Fig. 6B). A space separates the nasals from the oblique cartilage of the nasal capsule. The parasphenoid is T- shaped, not fused with the subjacent bones. The cultriform process, which rests on the sphenethmoid, is long not keeled and anteriorly serrated, their tips do not reach the level of the neopalatines. The posteromedial process of the parasphenoid is acuminate and near the foramen magnum margin. The alae deflect posteriorly and they gradually expand to the cartilaginous extreme. The neopalatines are curved bones, concave posteriorly. One-third of it inner length overlaps with the sphenethmoid. The outer edge reaches the pars palatine of the maxilla. The vomers are paired bones that overlap the sphenethmoid. Each vomer comprises a dentigerous process with a transverse and concave row that bears 4 to 5 teeth. The posterior margin forms the posterior margin of choanae. The maxillary arcade is complete. The upper jaw is composed of the premaxillae, maxillae and quadratojugal bones. Each premaxilla bears 4–5 teeth. The alary process is subrectangular, oriented dorsally curved backwards and not reach the nasals. The pars palatina is subrectangular and the palatine process is pointed. The maxilla is well-developed, pars fascialis is wide and pars palatina narrow. The teeth (19–24) are conical. Suspensorium: Each pterygoid has well-developed the anterior, medial and posterior rami. The anterior ramus is expanded anteriorly, articulating with the inner side of the maxilla. The medial ramus does not reach the allae of parasphenoid, but it contacts on the prootic. The posterior ramus invests the cartilaginous quadrate process medially and terminates posteriorly at the angle of the jaw. The zygomatic ramus of the squamosal is slightly curved (Fig. 6C). The otic ramus is expanded and shorter than zygomatic ramus. The ventral ramus is straight and the angle with the maxilla is about 45º. The annulus tympanic is oned dorsally, cartilaginous and joined to the zygomatic ramus. The coronoid process of the mandible is trapezoidal and normally developed. Columella presents. Hyoid apparatus: The hyoid plate is cartilaginous and mineralized at posteromedial processes (Fig. 6E). At midline the hyoid corpus is 1.2 times wider than long; the hypoglossal sinus is broad and U-shaped, approximately as deep as wide and its margins are parallel. The allary processes are thin, perpendicular to the axial axis of the hyoid plate, slightly oriented forward and with a distal expansion. The hyales are thin and curved; the anterior lateral processes are developed and slightly curved laterally. The postero-lateral processes are thin and oriented postero-laterally, their tips are sharp. The posterior ends of the posteromedial processes are cartilaginous and slightly expanded. Pectoral girdle: The pectoral girdle is arciferous, the omosternum is cartilaginous with an expanded distal end 60 · Zootaxa 3305 © 2012 Magnolia Press NUÑEZET AL. (Fig. 6D). The anterior expansion is shorter than the cartilaginous sternum. The omosternum slightly expanded anteriorly and stick-like. The sternum is wide and its distal end is rounded. The procoracoid is present and extends to the level of the internal extreme of the clavicle, the clavicles do not touch each other. The prolongation of the procoracoid extends between the clavicle and the scapula. In ventral view, the right cartilaginous epicoracoid overlaps the left. The pectoral fenestra, whose inner margin is concave is 1.5 times wider than long. Each of these apertures is anteriorly bordered by the procoracoid cartilage, medially by the epicoraciod cartilage, and posteriolly by the coracoid. Each clavicule is concave anteriorly. The glenoid end of the clavicle is expanded dorsolaterally into a wedge-shaped process that articulates with the pars acromialis of the scapula. The clavicles do not reach the glenoid fossa. The scapula is rectangular in shape and 1.3 times the coracoid. The scapula is composed of two planes: pars acromialis concave and the pars glenoid concave posteriorly. The coracoid is subrectangular and the distal ends are distally expanded. The glenoid cavity is limited by the pars glenoidalis and coracoid. The outer edge of the supra scapular cartilage is cartilaginous. The cleitrum consists of an ossified thin and bifid lamina, the posterior ramus is shorter of the anterior ramus slightly expanded, as long as the scapula; the anterior border ossified as cleitrum; the posterior and lateral margins unmineralized. FIGURE 6. Skull of Eupsophus altor sp. nov. (IZUA 3619); in dorsal (A),ventral (B), and lateral (C) views. Pectoral girdle (D) and hyoid plate (E) of Eupsophus altor sp. nov. Bar indicates 5 mm. Gray areas indicate cartilaginous tissue. Axial osteology: The vertebral columnae is composed of eight procoelus, non-imbricate, independent presacral vertebrae (Fig.7A, B). Presacral I (atlas) wide, shallow cervical cotyles widely separated. The presacrals II–IV with low neural spines, presacrals V–VIII with neural spine absent. Relative lengths of transverse processes and sacrum: III = sacrum < IV< II< V–VII<VIII. Distal ends of presacral III slightly expanded; transverse processes of A NEW SPECIES OF EUPSOPHUS FROM SOUTHERN CHILE Zootaxa 3305 © 2012 Magnolia Press · 61 presacrals V–VIII acuminated. Presacral II oriented anteriorly, III–VII oriented posteriorly and VIII oriented perpendicularly to the longitudinal axis. Sacral diapophysis rounded, end slightly expanded, oriented posterolateraly; sacrum with bicondylar articulation with urostyle. Urostyle robust bearing dorsal crest that is more developed anteriorly, approximately 1.2 times larger than sacrum plus presacral vertebral column. Overall length of pelvic girdle 1.6 times the length of sacrum plus presacral vertebral column. Ilial shaft poorly developed, interilial profile U-shaped, width of the U at the anterior ends of the ilia approximately 2.5 times its base. Ilium forming anterior margin of round acetabulum; preacetabulum forming approximately a 45º angle to the ilial shaft; ilia articulating with one another medially forming the anterior margin of acetabulum; ventral margin of acetabulum formed by cartilaginous pubis. The ischium is prominent, articulating with the ilium and fused with the pubis (Fig. 7C). FIGURE 7. Vertebral column of Eupsophus altor sp. nov. (IZUA 3619) in dorsal (A), and ventral (B) views. Left acetabulum of Eupsophus altor sp. nov. (C). Dorsal views of the hand (D) and foot (E) of Eupsophus altor sp. nov. Bar indicates 5 mm. (C1–C4= carpal bones; T1–T3 = tarsal bones). 62 · Zootaxa 3305 © 2012 Magnolia Press NUÑEZET AL.
