Characterization of a Cystine-Rich Polyphenolic Protein Family from the Blue Mussel Mytilus edulis L PDF
Preview Characterization of a Cystine-Rich Polyphenolic Protein Family from the Blue Mussel Mytilus edulis L
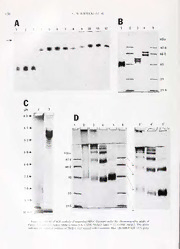
Reference: Biol. Bull. 183: 123-137. (August, 1992) Characterization of a Cystine-Rich Polyphenolic Protein Family from the Blue Mussel Mytilus edulis L.1 LESZEK M. RZEPECKI1 KAROLYN M. HANSEN, AND J. HERBERT WAITE , College ofMarine Studies. University ofDelaware, 700 Pilottown Road, Lewes, Delaware 19958 Abstract. Marine bivalve mollusks synthesize, in the the primary sequence of Mefp-2 is tandemly repetitive, phenol and accessory glands ofthe foot, proteins that in- with at leastthreetypesofmotif. Thesequencedegeneracy tegrate the post-translationally hydroxylated amino acid ofthe motifs is greater than in Mefp-1. Mefp-2 has min- 3,4-dihydroxyphenylalanine (DOPA) into their primary imal sequence homology with known structural proteins sequence. These polyphenolic proteins serveasstructural and may be a structural element ofthe plaque matrix. andadhesivecomponentsofthebyssal threadswhich form the extraorganismic holdfast. One family of byssal pre- Introduction cursors, previouslycharacterized in a numberofmytiloid species, consists of proteins between 70-130 kDa con- Mussels make a secure adhesive holdfast (byssus) that taining 8-18 mol % DOPA. The high molecular weight bonds tightly to wet and irregular surfaces without the precursor isolated from the foot ofthe blue mussel (My- extraordinary preparative treatments required when hu- tilus edulis Linnaeus, 1758) is here designated as Mefp- mans try their hand at similar tasks (Waite, 1987). The 1. We now present evidence for the occurrence, in M. byssus, acollagenousextraorganismictendon, isabundle DOPA edulis, ofasecond, structurally unrelated, familyof ofthreads that terminate in an adhesive plaque. The bys- proteins (Mefp-2) ofabout 42-47 kDa. These novel pro- sus is synthesized by a muscular organ, the mussel foot teinscontain 2-3 mol % DOPA and, in startling contrast (see schematic diagram in Fig. 1). The thread coalesces to Mefp-1, are also enriched in the disulphide-containing from the secretions of various glands lining the ventral amino acid cystine (6-7 mol %). Consideration of the groove of the foot, while the plaque forms at the distal amino acid compositions ofMefp-1 and 2 and ofthe ter- depression. The collagenous core ofthethread is secreted minal adhesive plaques of byssal threads suggests that by the collagen gland (Vitellaro-Zuccarello, 1980), and a Mefp-2 makes up about 25% ofplaque protein, whereas protective varnish, madeofan o-diphenolicresincoupled Mefp-1 content is about 5%. The Mefp-2 family exhibits with a curing enzyme, is secreted by the accessory gland electrophoretic microheterogeneity, but members share and applied to the thread cortex (Brown, 1952; Pujol, similar N- andC-terminal amino acid sequences. Analysis 1967; Vitellaro-Zuccarello, 1981). The resin is known to ofpeptides isolated after tryptic hydrolysis suggests that be a 130 kDa, tandemly repetitive protein (here called Mefp-1, Mytilus edulis foot protein), which incorporates into its primary structure a high proportion of3,4-dihy- ARbebcreeivvieadti2o7nsM:arDcOhPA1,9923;.4a-cdciehpytderdo.2x0ypMhaenyyl1a9l9a2n.ine; Mefp-1 & -2. droxyphenylalanine (DOPA), synthesized post- or co- Mytilusedulisfootprotein 1 and2;CAMC;S-carhamidomethylcysteine; translationally through the action ofa tyrosyl hydroxylase COMC, S-carboxymethylcysteine; NBA, Na/Borate/Ascorbate buffer: (Waite, 1983; Benedict and Waite, 1986a). The curing DTT.dithiothreitol:HPLC.highpressureliquidchromatography;EDTA, enzyme is a catecholoxidase, activated after secretion of ethylenediaminetetraacetic acid; SDS- or AU-PAGE. sodium dodecyl- the resin, which convertsthe peptidyl DOPA residues into esrluaelzcpothlraiictuemf.oorcuascseitnigc:aPciAdS-,urpeearipoodliycacarcyilda-mScirdueffgesltaeilne;ctNroBpTho,rensiilsr;oIbEluFe,itseot-- dpeuprtiindgylscDleOrPoAti-zqautiionno(nWea,itae,pr1o9b9a0b;leRzcerpoescsk-liinaknidngWaaigteen,t ' Correspondingauthor. 1991, for reviews). 123 124 L. M. RZEPECKI ET AL The plaque is formed from tjandular secretion at the In all ofthechromatography procedures, theseparation distal depression, : gland involved appears ofMefp-1 and Mefp-2 wasassessed bygel electrophoresis to be the phenol t .;!ied because of its intense (see below). Proteins were lyophilized after HPLC puri- affinity forphen histological reagents(Tamarin fication and stored dry at -20C. DOPA concentrations ela/.. 1976). ' s to Mefp-1 bind stronglytophenol were determined by the Arnow (1937) method (Waite and accessor} ids (Benedict and Waite, 1986a), and and Tanzer, 1981). and the protein concentrations were Mefp-1 !; Dilated from dissected phenol glands determined accordingto Bradford(1976),withaBio-Rad (Waite, 1983). Mefp-1 has also been identified as the ad- (Richmond, California) reagent kit. hesive at the interface between plaque and substratum The localization of Mefp-2 was studied in specimens (Benedict and Waite,- 1986a), and its physicochemical ofM. edulis that were collected locally (Roosevelt Inlet, properties are consistent with such a role (Filpula et al., Lewes, Delaware), kept in flowing seawater at 12-15C, 1990). and allowed to deposit byssal threads on to plexiglass Little isknown, however, about thecomponents ofthe plates. In one set ofexperiments, some mussel feet were plaque matrix. We have now characterized another po- excised and serially sectioned, as illustrated in Figure la. tential ingredientofphenolglandsecretion, Mefp-2, which The acid-urea soluble protein was extracted from each may be a structural element of plaque matrix. Mefp-2 section by homogenization in a small glass homogenizer incorporates some DOPA, but isdistinct from Mefp-1 in containing 200-300 n\ of5% acetic acid, 8 Al urea. The composition, sequence, and physicochemical properties, paniculate matter was sedimented at 13.000 x g for 5 and hence probably serves a different role in byssal struc- min and was then analyzed for acid-soluble proteins by ture. electrophoresis. In asecondsetofexperiments, byssi were collected 24 h after their synthesis and rinsed in water; Materials and Methods the plaquesand threadswerethenseparatedwithascalpel. About 1 10-140 mgand 150-175 mgwetweightofplaques Purification of DOPA proteins and threads, respectively, were homogenized, with a little silica powder (Silica Gel G, 250 //m Analtech, Newark, Cultured blue mussels(Mylilusedulis L.)wereobtained Delaware) tMo provide abrasion, in either400 /jl 5% acetic via Rehoboth Seafood Market (Rehoboth Beach, Dela- acid with 8 urea, or in 200^1 ofthe sample buffer used ware) after being shipped live from Maine on ice. Feet for SDS-PAGE (Laemmli, 1970); the particulate matter were excised within two days of original shipment and was removed asabove. Polyclonal anti-Mefp-2 rabbitan- stored in 30-g lots at -70C. tibodies were contractually prepared by Cambridge Re- The DOPA proteins were extracted and purified, as search Biochemicals(Cambridge, Great Britain), and were describedby Pardo etal. (1990) and modified by Rzepecki used to determine the immunoreactivity of thread and elul. (1991). Briefly, 30gofmussel feetwere homogenized plaque extracts and control Mefp-2 (Blake et al., 1984). in a Waring blendor in 300 ml 0.7% perchloric acid, and Dot blots on nitrocellulose were exposed for 1 h to a 10~5 the homogenate was sedimented at 31.000 X gto remove dilution of serum, or a 10 4 dilution of pre-immune paniculate matter. The DOPA proteins precipitated upon serum. Binding was detected with goat anti-rabbit IgG- the addition ofacetone to 66% final volume. The precip- coupled alkaline phosphatase (Boehringer-Mannheim, itate was resolubilized in 5-7 ml 5% acetic acid and then Indianapolis, Indiana). fractionated,inthesamebuffer, ona2.5 X 90cmcolumn ofSephadex G-200 or G-150 (Pharmacia LKB Biotech- S-A/ky/cition ofMefp-2 nology Inc., Piscataway, New Jersey). The eluate was monitored at 280 nm. The final purification of Mefp-2 S-Alkylation of Mefp-2 to S-carbamidomethyl-Mefp- was achieved as follows. The lyophilized Sephadex frac- 2 (CAMC-Mefp-2) orS-carboxymethyl-Mefp-2 (COMC- tions were chromatographed in 5% acetic acid on a 2.5 Mefp-2) was effected after the cystine residues had been X 90cm col ofSephacryl S-300 orS-400 (Pharmacia) reduced by a modification ofa procedure ofHollecker's monitored nm. This step was followed by high (1990). Stock solutionsofMefp-2 (10 mg/ml)were freshly pressure IR, ^matography (HPLC) on a 0.7 X 25 pMrepared in H2O. To 80 n\ Mefp-2 wasadded 20 n\ of0.1 cm semi-prepai RrownleeAquapore RP-300(C8)re- EDTA. pH 7.0, which caused precipMitation. After the vMearssseacphhuassetetsc)olwuith i'aininHIPnXstsroulmveennttdCeol.i,verWyosbyusrtne,m Maddaistcioornbiocfa4c0i0d,nb\roofugNhBtAtobpuHffe8r.0(0w.i5thM6bNorNicaOaHci)d., t0.h5e (Rainin); monitonrij- "SO nm with a Model 1 16 precipitate redissolved: 20 jul of fresh 1 dithiothreitol dualwavelengthdetecu, < iilson Medical ElectronicsInc., (DTT)togetherwith 1.15 mlof8 A/ureawerethenadded, Middleton, Wisconsin). The acetonitrile/water solvent and the reaction mixture was incubated for 40 min at gradients contained 0.1% trifluoracetic acid. room temperature. Afterthe reduction, the cyteines were MUSSEL CYSTINE-RICH DOPA PROTEINS 125 5-alkylated by the addition oMffresh iodoacetamide or io- or absence of 10% phenol. DOPA, tyrosine, serine, and doacetate (300 n\ ofa 0.25 stock in a 1:4 dilution of threonine values were recovered from phenolic hydroly- NBA the buffer for iodoacetamide, or, for iodoacetate, sates, while cystine and histidine values were obtained undiluted NBA buffercontaining0.6 j*lof6 TVNaOH per from aphenolic hydrolysates; we deduced a corrected mg iodoacetate), and the reaction mixture was incubated composition from both data sets using the lysine valueas fora further 30 min. The reaction was terminated by the a common factor. In the case of reduced and alkylated addition of20n\ glacial acetic acid, and the proteinswere Mefp-2, alkylated cysteine was recovered after hydrolysis immediately separated from the low molecular weight as S-carboxymethylcysteine (5-COMC) which co-mi- solutes by HPLC, as described. Control alkylations of gratedon theaminoacidanalyzerwith 3-hydroxyproline. Mefp-2 in buffers with urea, but no DTT, were also per- Because native Mefp-2 contained no 3-hydroxyproline. formed. Protein fractionswere lyophilized, redissolved in the content ofS-COMC was simply determined by ap- H2O. and stored at 4C for up to 1 week before use. plying a correction factor of 2.23 to the reported 3-hy- droxyproline values. This correction factor was empiri- Pcplidcpreparation andpurification cally determined by running a concentration series ofS- COMC Native Mefp-2 or its alkylated derivatives were incu- standards (Sigma) on the analyzer. Peptides and bated (in 100 m.M Tris, 100 mA/ ascorbate, pH 7.5, for proteins were sequenced on a microsequenator (Porton o7Mfanh1n:2ha5teiorrmo,1o:Im5n0ditfaoenrmap)ne,artaaittvuearater)nydpswaiilntkhytloatptrreyodptseMiinenfpw(-eB2io.gehhrtreisrnpagetecir-o smIonalsnvterdudmeaegnnrdtasdq,autaiTnoatnri.ztaaPntaTe,Hd-bCaaylmiHifnPoroLniCaac)aisdupsdrieenrvgiivoaautusitlvoyemsdaewtseecrdreiEbrdee-d- atiicnvicedulybt.aotMi5eo%fnpwf-ia2nsalctoevnrocmleiunnmtaerta,etdiaobnnydstthwheeeraepdedpaittbiiodouentso1fwegmlrgaec/imralels.aoclTevtheiedc f(mmWoAaMr/imteesd,odo1in9u94m1.)6.phmAogds/dpihmtalitoeMn,eafl1p8-N2-mtAien/rmasibonudaflifueramncabolonyrssaiitsset,iwnaagsnodfpe41r68- dpihraescetlycoblyuHmPnL(CRaoinnian)0,.4a6sXde2s5crcimbeMdiacbroovseo.rbInC1a8nreevfefrosret formesdodatiurmooamscotrebmapteer,atpuHre7f.o0r. dIinfcfeurbeanttiotnismeweinrteerpvearl-s to recover the DOPA peptides specifically, Mefp-2 (0.5 and the released amino acidsMwere analyzed directly. The comofgnt/thmaelia)nbiwonagvse0t.br4uefafmtelerdfowofirtp6hhhet,nrytylphseibnnolrioonan4dae0td0eo/aunlgoaafrsoamsae1l:l2(PdcBiolAlu-tu3im0on.n ahugsoaegidnksiitnd5nth0eiysmaNAm-/tiensroompdieinpuatmlidapanhsaoelsypshiast(weB.aosephpHrrien7p.ga0e,rreadtMabanynndohimaeilinysmai)ls Lot # JM-2134D with 37.8 ^Moles boron/ml; Amicon concentration of0.09 mg/ml. Corp., Danvers, Massachusetts) which had been equili- brated ina 1:5 dilutionoftheabovebuffer. Boundpeptides Gel electrophoresis andisoelectricfocussing werewashed twicewith0.4 ml ofthediluted buffer, twice more with 0.4 ml of deionized water, and eluted with Acetic acid-urea polyacrylamide gel electrophoresis athrraeteedwabsyhHesPLoCf.5% acetic acid. Eluted peptides were sep- (ChAaUl-klPMeAyGE( 1)96w9a)swiptehrf5o%rmpeodlyaaccrcyolradmiindge,to5%Paancyetiimc aacnidd and 8 urea, pH 2.7 (Rzepecki et al., 1991). Discontin- Amino acidanalysis andpeptide sequencing uoussodium dodecylsulphate polyacrylamidegel electro- phoresis (SDS-PAGE) was performed according to Lae- The amino acid compositions of the hydrolyzed pro- mmli (1970), except that various concentrations ofDTT teinsand peptidesweredetermined on a Beckman System were used in the sample treatment buffer. Low molecular 6300 Autoanalyzer (Beckman Instruments, Palo Alto, mass protein standards ranged from 14.4-97.4 kDa (Bio- California) using the ninhydrin reaction for detection Rad, California). Gelswere stained forprotein in 0.001% (Waite. 1991). Polypeptides were usually hydrolyzed by Coomassie Blue R-250 in 7.5% aceticacid, 40% methanol. the rapid method ofTsugita et a/., in 5 /V HC1 with 8% The DOPA proteins were visualized by staining the trifluoracetic acid and 8% phenol //; vacua at 158C for gelswith theArnow(1937) method, asdescribed byWaite 22 min, except that hydrolysis was effected in the bulk and Tanzer (1981). We also used a new redox cycling phase, rather than the vapor phase as in the original method involving the production of formazan from ni- method. Unfortunately, the high concentrationsofphenol troblue tetrazolium (NET) in the presence ofglycine at required for quantitative yields of DOPA caused the re- pH > \0(PazetaL, 1991), butatasmallcostinsensitivity, action ofphenol and cystinetogivean unidentified prod- did not usually transfer the proteins electrophoretically uct which co-migrated with histidine on the amino acid to nitrocellulose asrecommended bytheauthors. ForAr- analyzer. Consequently, the amino acid compositions of now assays, SDS gels were first acidified by equilibration native (non-alkylated) Mefp-2 were determined after hy- in 5% acetic acid; for NBT stain, all gels were washed N drolysis in 6 HC1, for 24 h at 105C. in the presence twice in 50-100 ml of0.2 A/ sodium borate, pH 8.5, to 126 L. M. RZEPECKI ET AL AG VG DD 4' 5' Figure 1. LocalizationofMefp-2 inMytiluscciitli\tootandhyssus.(A)AU-PAGEanalysisofacid-urea soluble proteins from aserially sectioned mussel foot. Lanes 1-5 correspond to the indicated footsections and were stained with Coomassie Blue. Lanes l'-5' correspond to the same sections run on a parallel gel stained by the NBT redox cycling assay. The drawn parallel bars indicate position ofMefp-1 (upper) and Mefp-2 (lower) proteins. Schematic codes are: S, stem ofthe byssus: CG. collagen gland; AG. accessory gland;VG,ventralgroove;DD.distaldepression;andPG.phenolgland.(B)SDS-PAGE(151? polyacrylamide) ofproteinsextractedfromthreadsandplaquesinSDSandneutralbuffer.Gelsstainedasindicated;symbols P. T, M-2 and St refer to: plaque extract, thread extract, pure Mefp-2, and molecular weight standards, respectively;arrowsindicate Mefp-2. (C)AU-PAGE analysisofaceticacid-ureasolubleproteinsfrom threads and plaques. Gelsstainedasindicated:symbolsareasin(B);arrowsindicateMefp-2. Thehighermolecular weight aggregates apparent in pure Mefp-2 in (B) and (C) were artefactual results ofprolonged storage in waterat 20C and were never found in freshly isolated protein. Arnow tests were not performed in (B) and (C). (D) Dot blots ofpure DOPA proteins, and thread and plaque acetic acid-urea soluble proteins, stainedasindicated. Theamountoftotal protein blotted isgiven in thecenter: symbolsareasin (B). remove the electi is buffers before addingthe NBT borate and then in 5% acetic acid. Unless specified, how- redoxcyclingreageni nftheNBTredoxcyclingassay ever, the presence of DOPA in electrophoretic protein was prefered in this nee, though it is somewhat species was confirmed at least once by the Arnow stain lessspecificthantheAnn iy indetectingo-diphenols, before routine use ofthe NBT assay. it is considerably more scnsune. and the stain lasts in- Glycoproteinsweredetectedbytheperiodicacid-Schiff definitely, oncethegels have been washed first in alkaline (PAS) stain (Segrest and Jackson, 1972), with bovine a,- MUSSEL CYSTINE-RICH DOPA PROTEINS 127 B P T M-2 St p T M-2 P T M-2 P T M-2 21-5 14-4 Coomassie NBT Coomassie NBT M-2 M-2 |ig Protein 1-0 0-1 001 001 NBT Anti-Mefp-2 Figure 1. (Continued) acid glycoprotein (Sigma Chemical Co., St. Louis, Mis- Results and Discussion souri) and collagen (type II calfskin) serving as positive controls. Localisation ofMefp-2 in M. edulis byssus Denaturing isoelectric focussing (IEF) was performed with pH 3-10 amMpholytes(FMC Bioproducts, Rockland, DOPAproteinsextracted from seriallysectioned mussel Musairnygla0n.d02) Min 8aceticuraecai,d1a0n%d g1lyNcerNola,O6H%atsritthoenaXn-o1l0y0t,e dfeeettecatnedd eblyectthreopNhoBrTetirceadlolyx rceyscollivnegdasbsyayAUan-dPAwiGtEh Cwoeor-e andcatholyte, respectively(Guilian ctal.. 1984). Gelswere massie Blue (Fig. la). Mefp-1 migrated asadoublet iden- pre-equilibrated (200 V, 15 min; 500 V, 30 min; 800 V, ticaltothepreviouslycharacterized 130kDDOPA protein 30 min) before samples (in the above buffer with 15 mg/ (Waite. 1983), and it was found in foot sections through- ml DTT) were applied to the gel under the anolyte. Pro- out the length ofthe ventral groove corresponding to ac- teins were focussed for 2-3 h at 1000 V, and a lane was cessory and phenol glands. Mefp-2 was exclusively asso- removed for pH gradient determination (by incubation ciated with the distal foot section that encompasses the of0.5 cmgel slicesovernight in 1 mlof0.' 770- NaCl)before phenol gland involved in plaque secretion. Several minor the gel was stained. NBT-positive proteins of intermediate electrophoretic 128 L. M. RZEPECKI ET AL. orG-200 in 5% acetic acid (G-200 gave better separation but was not reuseable). Mefp-2, the predominant com- ponent of the trailing Sephadex fractions, was rechro- matographedon Sephacryl S-300orS-400, because HPLC alone failed to separate Mefp-2 from residual Mefp-l. Al- though the difficulty ofassessingthe relativecontributions ofeither DOPA protein toan unknown mixture preclude presentation of a quantitative purification table, final yields ofpure Mefp-2 ranged between 1-5 mg from 30 g 20 40 60 wet mussel feet, depending on the season ofthe year in which the mussels were collected (mussels collected in Elulion time (mini winter gave better yields than in other seasons). Mefp-2 Figure 2. HPLC chromatography profiles ofMefp-2 (- and was resolved by AU-PAGE into two predominant elec- Mxef2p5-lcm( semi-1pmroenpiatraotrievdeaCt82c8o0lnumm.n wPirottheiannsawceertoenirtersiollevgerdadoinenat,0.a7s trophoretic bands (Fig. 2, inset), although minor bands indicatedbytheinclineddashedline.Inset:AU-PAGEofpunnedMefp- 2 at three concentrations, stained with Coomassie Blue. The arrow in- dicatesthepositionoftrace Mefp-l contaminants. Table I Ammoacidcomposition ofMefp-2 andrelatedproteins' mobility were also observed in this distal segment, but their origin and nature were not investigated further. To Amino acid. obtain a better separation between phenol and accessory glands, we micro-dissected phenol and accessory glands from thin foot sectionsand again, Mefp-2 wasexclusively detected in the phenol gland, whereas Mefp-l appeared in both glands. No other byssal glands are known to con- DOPA tain proteins. Extraction ofbyssal plaques in either acetic acid-urea or neutral SDS buffers yielded a polypeptide that co-mi- gratedwith Mefp-2 duringboth SDS-and AU-PAGE and NET stained positively with (Fig. Ib. c): miscellaneous electrophoretic species that stained with Coomassie Blue, but not NBT, also appeared. Preliminary amino acid analysis following HPLC purification ofthisNBT-positive plaque polypeptide revealed a composition very similar to authentic Mefp-2 (Diamond and Waite. unpub.). No polypeptideextracted from byssalthreadsaloneexhibited NBT reactivity orelectrophoretic behaviorakin to Mefp- 2, and no polypeptides corresponding to intact Mefp-l weredetected in plaque orthread extracts. Dot blotscon- firmed that a considerable proportion (ca. 10%) ofacid- soluble plaque protein was NBT-sensitive. and immu- noreactivity assays demonstrated that the extracted ma- terial that reacted with anti-Mefp-2 antibodieswasspecific to plaques (Fig. Id). Pre-immune serum was unreactive in these assays. These observations are complicated by some cross-reactivity between the as yet unpurified poly- clonal anti-Mi i 1 and pure Mefp-l, but the electropho- resis and dot bl; nits together strongly suggest that, because Mefp-i \ed to occur in both threads and ! plaques (Benedict Vaite, 1986a), Mefp-2 is an im- portant component si ''the plaque. and Purification coni/>. \lcfp-2 Considerable separation ofMefp-l and -2 wasobtained by chromatography of the proteins on Sephadex G-150 MUSSEL CYSTINE-RICH DOPA PROTEINS 129 also found in Mefp-2, in addition to othersignificant dif- ferences. Acomparison ofthe Mefp-1, Mefp-2, andbyssal plaque amino acid compositions allows a crude estimate ofthe contributions of the two DOPA proteins to the overall plaque composition. Assuming that Mefp-1 is the only source of 3-hydroxyproline in byssus (Waite, 1983), the 100 amount ofMefp-1 in the plaque may be estimated from the plaque 3-Hyp value (Benedict and Waite, 1986b) to 80 be 4-5 mol % ofplaque protein, and the proportion of o DOPA residues belonging to Mefp-1 can be calculated. c 60 'C On the further assumption that all the remaining DOPA CO belongs to Mefp-2. a round figure of 25 mol % may be 40 calculated for the plaque content ofMefp-2. At this pos- tulated concentration, Mefp-2 would account for about 20 90% ofplaque cystine and 70% ofplaque lysine residues. Although these precise numbers should be taken cum granosalts[since(i) 3-Hypoccursat low levels(<0.2 mol 20 40 %) in the plaque and isdifficult toquantitate; (ii) thecon- DOPA Elution Time (min) tent ofthe readily oxidized residues is easily un- Figure 3. HPLC chromatography profiles of control Mefp-2 derestimatedD,OePveAn in fresh byssus;and (iii) minorproteins andCAMC-Mefp-2 andCOMC-Mefp-2 containing or 3-Hyp may occur], they are consis- ), ( ) ( ), reducedandalkylatedasindicatedin Materialsand Methods,andmon- tent with the apparent proportion of Mefp-2 extractable itored at 280 nm. Proteins were resolved on a0.46 x 25 cm analytical from plaque and foot (Fig. 1). These considerations thus C8columnusingthesamegradientprogramasshowninFigure2(inclined establish an upper limit to the content of Mefp-2 in the dashed line). Concentration ofcontrol Mefp-2 was halfthat ofthe al- plaque. kvlated derivatives, but thedetectorattenuation washalved. HPLCand electrophoretic characterization ofMefp-2 ofsimilar mobility were often apparent. All reacted pos- itivelywith the NBTand odiphenol-specific Arnow stains HPLC and electrophoretic analyses of native, as well (not shown), consistent with presence of DOPA in the asreduced andS-alkylated Mefp-2 revealedachallenging amino acid composition. Mefp-2, resolved by SDS-PAGE complexity in chromatographic and electrophoretic be- (see below), did not stain with the PAS procedure forcis- havior. The alkylated Mefp-2 derivatives eluted more diol sugars, though control proteins in the same gel were slowly than control protein on HPLC, with the S-car- PAS-positive, suggesting that Mefp-2. in common with boxymethylated protein (COMC-Mefp-2) unexpectedly Mefp-1, is not a glycoprotein. the slower ofthe two (Fig. 3). Mefp-2 that had been re- The most dramatic compositional difference between duced with DTT, but not alkylated, eluted with a delay Mefp-1 and -2 (Table I) is the occurrence ofcystine ex- comparable to COMC-Mefp-2 (not shown). Denaturing clusively in Mefp-2 and at remarkably high levels (6-7 AU-PAGE profiles of fractions under the chromato- mol%). Acomparison ofnative Mefp-2 with reducedand graphicpeaksofFigure 3 showedthatthetwo majorbands S-carbamidomethylated protein (CAMC-Mefp-2), to- exhibited by native Mefp-2 had been converted, upon re- gether with the exceedingly low levels of S-carboxyme- duction and alkylation, into multiplets with fouror more thylcysteine in the acid hydrolysatesofnative MefpM-2 that components (Fig. 4a). Both COMC- and CAMC-Mefp-2 had been treated with iodoacetamide in 4.8 or 6 urea migrated considerably more slowly than the native poly- without prior reduction by DTT, shows that most ofthe mer, with COMC-Mefp-2 again the slower of the pair. S-alkylated cysteine (ca. 15 mol %) had originally been Mefp-2 that had been reduced by DTT, but notalkylated, in thedisulphide form. The DOPA contentofMefp-2 (2- migrated with intermediate mobility, but upon storage in 3 mol %) was much lowerthan that ofMefp-1 (11-18%) water at 4C, partially regained the faster mobility char- and, unlike other molluscan DOPA proteins (Rzepecki acteristic ofnative Mefp-2 (not shown). Allelectrophoretic ct ui. 1991), the Lys:DOPA ratio farexceeded unity. Both species stained positively for DOPA with both NBT and contained proline, but no hydroxylation to 3- and 4-Hyp Arnow tests (not shown). occurred in Mefp-2. Glycine, rare in Mefp-1, made up Electrophoretic mobility on denaturing AU-PAGE about 14 mol % ofMefp-2. Elevated levels (about 5-fold) systems depends both on molecular weight and charge of Asx and Glx, and lower levels of Ser and Thr, were density (Hollecker, 1990). Although alkylation of Mefp-2 130 L. M. RZEPECKI ET AL. B A 12345 1 5 6 7 8 9 10 11 12 kDa 97-4 66-2 * 45 * 31 21-5 c pH 1 2 3' 5' 4-7 w^^^*^ -*-*^^ 5-3- 5-9- kDa 97-4 6-6> \ 66-2 \ 7-1- 45 7-6* 31 i 21-5 10 Figim (M AU-PAGE analysis ofsequential HPLC fractions under the chromatographic peaks of Figun i -3, native Mefp-2; lanes 4-8. CAMC-Mefp-2; lanes 9-12, COMC-Mefp-2. The arrow indicates tl il position ofMefp-l. Gel stained with Coomassie Blue. (B) SDS-PAGE (12% poly- MUSSEL CYSTINE-RICH DOPA PROTEINS 131 would increase the molecular mass of the protein by lytes), similar to that for Mefp-1. CAMC-Mefp-2, which some 3-3.5 kDa (assuming about 56-62 alkylated cys- theoretically has the same net charge as native Mefp-2, teines per protein of42-47 kDa with 360-400 amino ac- migrated identically (not shown). COMC-Mefp-2, with a ids), this increase alone seems insufficient to account for greatly decreased net positive charge owing to the addi- the reduced mobility under AU-PAGE, so modified tional carboxyl moieties, migrated as a family ofat least charge density and alterations in protein conformation ten distinct members, with pi values in the range4.7-5.9. inducedbydisulphide reduction andalkylation undoubt- Analogous electrophoretic heterogeneity occurs in the edly contributed to the electrophoretic differences. The DOPA proteins of Fasciola hepatica (Waite and Rice- overall similarity between the relative mobilities of Ficht, 1989). CAMC- and COMC-Mefp-2. which havewidelydifferent The variable migration ofnative Mefp-2 during SDS- pi values (see below), togetherwith their common differ- PAGE caused us to investigate further the effects ofDTT ence from native Mefp-2. which has the same pi as on electrophoretic behavior in thissystem. In theabsence CAMC-Mefp-2, argue for the pre-eminence of confor- of DTT, Mefp-2 could barely be induced to enter even mational changes. A transformation from a compact to thestackinggel ofdiscontinuous SDS-PAGE systems(Fig. anextended configuration upon reduction andalkylation 4d). As the DTT concentration was increased to 50 mA/, is consistent with the observed anomalous electrophoretic progressively more Mefp-2 entered the resolving gel and and chromatographic migration. migrated in a ladder-like pattern consistent with the pres- AnalysisofMefp-2 and itsalkylatedderivativesbySDS- ence of higher molecular weight aggregates of2, 3, 4 or PAGE. following reduction in 50 mAlDTT, wassimilarly more polypeptide chains. At the highest DTT concentra- complex (Fig. 4b). In contrast to Mefp-1. which precipi- tions, most ofthe Mefp-2 migrated asan apparent mono- tates in SDS, native Mefp-2 usually exhibited two elec- mer (cf. Fig. 4b). The obvious explanation for this obser- trophoretic bands (at low loading concentrations) with vation, that the oligomeric Mefp-2 resulted from inter- apparent molecular masses in the range 42-47 kDa, al- moleculardisulphide bonds, does not seem tenable, since though minor NBT-positive bands migrating in the same significant high molecular weight aggregates were rarely range were frequently present. Electrophoretic migration observed upon resolution ofthesameprotein preparations was, however, somewhat variable within that range and by denaturing AU-PAGE (although they sometimes oc- appeareddependenton theproteinconcentration andde- curred on prolonged storage when frozen in water cf. gree of reduction. Several electrophoretic bands [which also Figs. Ib, c and 2, with Fig. 4a, d). Apparently, then, exhibited positive NBTand Arnow reactions(not shown)] conditions exist forthe aggregation ofnative, but not re- were resolved for CAMC- and COMC-Mefp-2, with ap- duced, Mefp-2 in solution, although the precise contri- parent molecular masses in the ranges 48-54 kDa and bution, ifany, ofSDS to this phenomenon remains to be 63-71 kDa, respectively. The decreased mobility of determined. This is consistent with the precipitation fre- CAMC-Mefp-2 was almost, yet not quite, explained by quently noted in concentrated neutral solutions ofMefp- the molecular mass increment (3-3.5 kDa, see above) re- 2,andwith preliminary X-rayscatteringdata(Trumbore. sulting from alkylation, but COMC-Mefp-2 migrated pers. comm.). considerably more slowly. 5-Alkylation with iodoacetate iswell known to induceelectrophoretic anomalies(Lane, Primary structure ojMefp-2 1978). Isoelectric focussing (IEF) studies in highly reducing Despite the apparent electrophoretic heterogeneity of and denaturing media (Fig. 4c) showed that the apparent Mefp-2, N-terminal sequencing ofpure protein revealed pi ofnative Mefp-2 lay between 9 and 10 pH units (i.e., a single detectable N-terminus, HiN-Thr-Ser-Pro-Xaa- largely beyond the resolving power ofavailable ampho- Yaa-Dop-Asp-Asp-Asp-Glu . . . , where Xaa and Yaa acrylamide) analysis of Mefp-2 and derivatives. Lane 1. native Mefp-2; lane 3, CAMC-Mefp-2; lane 4, COMC-Mefp-2; lanes 2 and 5, molecular weight standards. Gel stained with Coomassie Blue. (C) IEF analysisofMefp-2. Lane 1. native Mefp-2; lane 2. COMC-Mefp-2; CAMC-Mefp-2 migrated identically to nativeMefp-2andisnotshown. Proteinswerefocussedonseparategelsinparallel,andthegelswerestained with the NBT redox cycling assay. The pH gradient is indicated on the left. (D) EffectsofDTT on native Mefp-2migrationduringSDS-PAGE(12% polyacrylamide). Lanes I and6aremolecularweightstandards. DTT concentrations: lane 2, m,U; lane 3, 0.5 mAl; lane 4, 5 mA/; lane 5. 50 mA/. The gel was stained with Coomassie Blue. Lanes 3'-5' are from a gel run in parallel, but stained with the NBT redox cycling assay, and correspond tolanes3-5 inthegel on the left. 132 L. M. RZEPECKI ET AL were predominantly 1: ' l, though Tyr. Gly, Thr leavingthe residual disulphide bonded coreprotein largely andGin wereals<i Table II). Treatment ofMefp- intact. 2 with aminopc ceased low levels ofThr, Ser, Peptidesobtained byHPLCchromatographyoftrypsin- Pro. DOPA < unsistent with the sequenator treated native and alkylated Mefp-2 fell generally into analysis. 1 al combination ofvariability and threemajorclasses,allcontainingeithertyrosineorDOPA constanc;. N-terminus might beexplicable in terms (Figs. 6, 7). All three types were isolated from alkylated mRNA ofmultiplegenesoralternate splicingmechanisms Mefp-2,butonlyTypeII peptideswereidentifiedindigests (c7. Bobek el al., 1988; Pihlajaniemi and Tamminen, of native Mefp-2. Owing to the chromatographic com- 1990),butanalysisofMefp-2 from an individual organism plexity ofthe peptide mixture, the relative content ofthe has not yet been possible. three classes was rather difficult to determine with any Native Mefp-2 (unlike Mefp-1) was relatively resistant precision. Most fractions contained two major peptides toavarietyofproteases, includingtrypsin, chymotrypsin, (usually in unequal proportions), and sequences for pep- pepsin. Staphylococcus aweus V8, and collagenase, even tides in various HPLC fractions were assigned by consid- at unusually high protease:Mefp-2 ratios (up to 1:1) in eration ofrelative yields ofamino acids in successive se- the presence ofurea, although increases in electrophoretic quenator cycles. Fortunately, sequence consistencies be- mobility and heterogeneity were often apparent upon came readily apparent, and aconsideration ofFigures 5- electrophoresis (see Fig. 5a, inset, for AU-PAGE oftryp- 7, assuming that UV absorbance at 230 nm broadly re- sin-treated Mefp-2). SDS-PAGE in the presence ofDTT flectspeptideconcentration, suggeststhatthe majority(at showed that, upon trypsin treatment, the apparent mo- least 80%) of Mefp-2 can probably be accounted for by lecular massdecreased from about 45 kDa to 30-35 kDa the identified motifs. with some increase in heterogeneity (not shown). HPLC Type I peptides. This type of motif appeared to pre- of trypsin-treated native Mefp-2 resolved some minor dominate in Mefp-2, with identified peptides accounting peptides (Fig. 5a), but reduction and alkylation of the for perhaps 40-50% ofthe 230 nm absorbance ofFigure residual disulphide bonded protein did not result in the 5b. although no individual peptide contributed more than recovery of any new peptides upon rechromatography, 5-10%. It was highly enriched in Cys, Gly, and Pro, and confirming that little internal nicking had occurred. was basic due to the excess ofLys and Arg over Glu and Trypsin digestion of both COMC- and CAMC-Mefp-2 Asp. Significantly, we found noevidence thatthe Tyrres- converted both alkylated derivatives to their component idues were hydroxylated to DOPA in this type ofmotif. peptides (Fig. 5). These results suggest that trypsin For convenience, we have subdivided the Type I motif trimmed native Mefp-2 at the N- orC-terminus, orboth. into six submotifs (Fig. 6), which combine variously to Table II \-lcmiiiiulanalysisulMe/p-2* Sequenatoranalysis Aminoacid (yield, pMoles x 10-')
