Changes in Salmon GnRH and Chicken GnRH-II Contents in the Brain and Pituitary, and GTH Contents in the Pituitary in Female Masu Salmon, Oncorhynchus masou, from Hatching through Ovulation(Endocrinology) PDF
Preview Changes in Salmon GnRH and Chicken GnRH-II Contents in the Brain and Pituitary, and GTH Contents in the Pituitary in Female Masu Salmon, Oncorhynchus masou, from Hatching through Ovulation(Endocrinology)
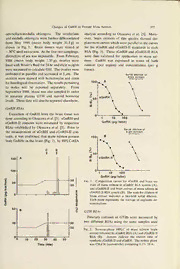
ZOOLOGICAL SCIENCE 9: 375-386 (1992) © 1992Zoological SocietyofJapan Changes in Salmon GnRH and Chicken GnRH-II Contents in the GTH Brain and Pituitary, and Contents in the Pituitary in Female Masu Salmon, Oncorhynchus masou, from Hatching through Ovulation Masafumi Amano, katsumi Aida, Naoto Okumoto1 and Yoshihisa Hasegawa2 Department ofFisheries, Faculty ofAgriculture, The University ofTokyo, Bunkyo, Tokyo, Japan, 1Nikko Branch, National Research Institute ofAquaculture, Chugushi, Nikko, Tochigi, Japan, 2Department ofObstetrics and Gynecology, Gunma University, School ofMedicine, Maebashi, Gunma, Japan — ABSTRACT Changes in salmon gonadotropin-releasing hormone (sGnRH) and chicken GnRH-II (cGnRH-II) contents in the brain and pituitary, and gonadotropin (GTH) subunits GTH I/?and GTH 11/3 contents in the pituitary of female masu salmon (Oncorhynchus masou) were investigated from hatching through ovulation. Gonadosomatic index (GSI) showed a gradual increase until the second summer(twoyear-olds),andthereafterarapidincreasewasobservedinaccordancewithvitellogenesis. Ovulationoccurredin autumn. BrainsGnRHwasalreadymeasurable athatching, whereascGnRH-II was first detected two months later. Both GnRHs contents increased during the underyearling phase, andfluctuatedthereafter. PituitarysGnRHcontentsshowedastepwiseincreaseeverysummerforthree years. sGnRH concentrations in each discrete brain area showed seasonal changes: high during autumn-winter and low in summer. sGnRH concentrations in the olfactory bulbs and telencephalon, and pituitary contents of sGnRH, GTH I/? and GTH II/3 significantly increased prior to ovulation. Pituitary GTH I/?contents showed clear seasonal changes for three years-high in autumn and low in winter-regardless ofthe state ofovarian maturity. Brain cGnRH-II contentswere lower than sGnRH contents. Moreover, pituitary cGnRH-II contents were undetectable and no significant changes in concentration in discrete brain areas were observed during vitellogenesis and ovulation. These results suggestthatsGnRHisinvolvedinovarianmaturationviatheregulationofGTHsynthesisandreleasein this species, whereas cGnRH-II has little or no involvement in reproduction. INTRODUCTION andin vitrounderexogenous administration [2,3], their physiological roles in the brain and pituitary Recent studies have shown that more than one are not fully understood. These two types of type of GnRH exists in the teleost brain [1]. In GnRHsexistnotonlyinthe hypothalamusbutalso most teleost species examined including salmo- in the other parts ofthe brain, and show differen- nids,salmonGnRH (sGnRH) andchicken GnRH- tial distributions [4, 5]. Furthermore, distribution II (cGnRH-II) have been detected. It is generally patterns of both GnRHs are vary according to accepted that one ofthe important roles ofGnRH species. Both sGnRH and cGnRH-II exist in the is the regulation of the synthesis and release of goldfish pituitary [4], but only sGnRH is detect- GTH by the GTH cells in the pituitary. Although able in rainbow trout Oncorhynchus mykiss pitui- both sGnRH and cGnRH-II molecules stimulate tary [5]. GTH thereleaseof inthefishpituitarybothin vivo There are few studies on the changes in brain GnRH contents in relation to gonadal maturation, Accepted November 25, 1991 and such results are not consistent. Gentile et al. Received October 14, 1991 [6] measured GnRH concentrations in the tel- 376 M. Amano, K. Aida et al. encephalon and hypothalamus of Venezuelan this study were offspring of wild fish which had freshwater fish, Pygocentrus notatus, using a migrated to the Shiribetsu River (Hokkaido). mammalian GnRH RIA system and found that Wild masu salmon migrate to the sea in the spring GnRH concentrations were high in mature fish. (1.5 years-old), andreturntotheriverinMayafter Yu et al. [7] measured GnRH content in discrete a one-year stay in the sea; they spawn in autumn brain areas offemale goldfish at different stages of anddie. Themasusalmonkeptintheinstitutealso ovarian development. However, brain GnRH smoltified at 1.5 years-old and matured at three content did not show clear parallel changes with years of age in fresh water, although the growth seasonal ovarian development. Okuzawa etal. [5] rate was not very rapid. There is a landlocked measured sGnRH and cGnRH-II contents in dis- form of masu salmon called "yamame". This crete brain areas of rainbow trout sampled in variety for the most part does not smoltify. September, and found that the pattern of sGnRH Sampling distribution differed with age and stage of sexual maturity. Samplingwascarriedoutonce amonth (twice in Recently, Suzuki et al. [8] reported that two May 1988) from December 1987 (month ofhatch- structurally different GTHs, GTH I and GTH II, ing) through October 1988, and at 2-4 months exist in the chum salmon pituitary. GTH I is intervals from January 1989 through October 1990 consideredtobeinvolvedinregulationoftheearly (month ofovulation). On the day ofautopsy, fish stages of gonadal maturation, and GTH II is were randomly selected and were anesthetized in considered to mainly regulate ovulation and sper- ethyl-p-aminobenzoate (0.05%). After measure- miation [9]. There is, however, no information on ments on body length and weight, brain and pitui- the changes in pituitary GTH I and GTH II tary were rapidly removed and frozen on dry-ice contentsthroughout the fishes' life span. Since the intended for the measurement of GnRHs and release of GTH is regulated by GnRH, it is GTHs. At the firsttwo samplings (December 1987 speculated that changes in pituitary GTH contents andJanuary 1988, meanbodyweight0.16-0.39g), are correlatedwith those inbrain GnRH contents. the head region was cut off and used for only Therefore, in the present study, we investigated GnRH measurement as dissection ofthebrainwas changes in sGnRH and cGnRH-II contents in the difficult. The pituitary was removed on sampling brain and pituitary, and GTH I/? and 11/? subunit occasions starting from April 1988 (mean body contents in the pituitary of female masu salmon weight 3.21 g). From September 1988 (mean body (Oncorhynchus masou) which were maintained in weight 17.2g), brain tissue was dissected into five fresh water for three years. Changes were fol- parts: the olfactory bulb, the telencephalon, the lowedfromhatchingthroughovulation, inorderto hypothalamus, the optic tectum-thalamus and the obtain basic information on annual rhythms of these hormones. MATERIALS AND METHODS Fish Forpurposes ofthisstudy, eggsofmasusalmon, Oncorhynchus masou, were artificially fertilized in October 1987 at the Nikko Branch, National Re- Fig. 1. Schematic diagram of sagittal section of masu search Institute of Aquaculture, Tochigi Prefec- salmon brain. Letters represent the followingbrain ture. Theeggshatchedin December 1987, andthe areas: a, olfactory bulbs and tracts; b, telencepha- lon, including preoptic area; c, hypothalamus; d, fish were reared under natural photoperiod in optic tectum-thalamus, including anterior part of spring water of constant temperature (9-10°C) cerebellum; e, cerebellum; f, medulla oblongata; g, throughout the experiment. The fish we used for pituitary. Changes of GnRH in Female Masu Salmon 377 cerebellum-medulla oblongata. The cerebellum analysis according to Okuzawa et al. [5]. More- and medulla oblongata were further differentiated over, brain extracts of this species showed dis- from May 1989 (mean body weight 34.5 g) as placementcurveswhichwere paralleltothe curves shown in Fig. 1. Brain tissues were stored at for the sGnRH and cGnRH-II standards in each —30°Cuntilextraction. Atthefirsttwosamplings, RIA (Fig. 3). These sGnRH and cGnRH-II RIA distinction ofsex was impossible. From February were thus validated for application to masu sal- 1988 (mean body weight 1.35 g), ovaries were mon. GnRH was expressed in terms of both fixed with Bouin's fluid for24hr and theirweights content (per region) and concentration (per g weremeasuredtocalculate GSI. The ovarieswere tissue). embedded in paraffin and sectioned at 5jum. The Serial dilution of sections were stained with hematoxylin and eosin brain extract forhistologicalobservation. Theresultspertaining 100n to males will be reported separately. From September 1988, blood was also sampled in order to measure plasma GTH and steroid hormone 50 levels. These datawill alsobe reportedelsewhere. GnRH RIAs Extraction of GnRH from the brain tissue was done accordingto Okuzawaetal. [5]. sGnRH and 1 10 100 cGnRH-II contents were measured by respective GnRH (pg/tube) RIAs established by Okuzawa et al. [5]. Prior to Serial dilution of the measurement of sGnRH and cGnRH-II con- brain extract tents, it was confirmed that masu salmon possess 100i both GnRHs in the brain (Fig. 2), by HPLC-RIA C S 1 1 50 150- A cGnRH-II 100- 1 10 100 GnRH (pg/tube) 50- 30 Fig. 3. Competition curves for sGnRH and brain ex- 20 tract of masu salmon in sGnRH RIA system (A), and cGnRH-II and brain extract ofmasu salmon in Q. o- _, rJLnji.-1—r- cGnRH-IIRIAsystem(B). Thescalefordilutionof brain extract indicates a two-fold serial dilution. I Each point represents the average of duplicate de- ECC terminations. (3 £ GTH RIAs Pituitary contents of GTHs were measured by two different RIAs using the same samples used Fig. 2. Reverse-phase HPLC of masu salmon brain extractfollowedbysGnRHRIA(A)andcGnRH-II RIA (B). Arrows indicate the elution time of 10 20 30 40 50 syntheticcGnRH-IIandsGnRH. Themobilephase Time (min) was CH3CN (acetonitrile) containing 0.1% TFA. 378 M. Amano, K. Aida et al. for GnRH measurement. GTH I/? and GTH 11/? tube (n=8). The antiserum against GTH 11/?was and antisera against GTH I/? and GTH 11/? were found to cross-react with GTH I, GTH II and kindly provided by Dr. H. Kawauchi of Kitasato GTHI/?at0.22%, 3.7% and 1.0%, respectively, at University. GTH 1/3and GTH 11/?were measured 50%) binding. by respective RIAs. GTH I/? and GTH 11/? were purified from chum salmon (Oncorhynchus keta) Statistics pituitary by Suzuki et al. [10], and the antisera The Student's Mest and Cochran-Cox test were against GTH I/? and GTH 11/? were raised by employed in statistical analysis. Suzuki et al. [9]. GTH I/? and GTH 11/? were iodinated according to the methodofKobayashiet RESULTS al. [11]. The procedure ofeach RIAwas the same as that in the sGTH RIA [11]. Displacement GSI curves for pituitary samples were parallel to the standard curves in both GTH I/? and GTH 11/? Changes in body weight and GSI are shown in RIA. The intra- and inter-assay coefficients of Fig. 4. GSI gradually increased from 0.18% variation in GTH I/? RIA were 10.9% (n=4) and (February 1988) to 0.52% (May 1990), showing a 23.0% (n=4), respectively, at about50% binding. small peak in September 1989. GSI rapidly in- The sensitivity of the assay, defined as twice the creased from July (0.73%) through October 1990 standard deviation at zero dose, was 62.5 pg/tube (12.6%), in accordance with the advancement of (n=5). The antiserm against GTH I/? cross- vitellogenesis and ovulation. Ovulation was reacted with GTH I, GTH II and GTH 11/? at observed in 9 out of 12 individuals in October 1.7%, 4.0% and 4.4%, respectively, at 50% bind- 1990. ing. The intra- and inter-assay coefficients of GTH variation in GTH 11/?RIAwere 12.0% (n=4) and Pituitary contents 11.7% (n=4), respectively. Sensitivity was 5 pg/ Changes in pituitary GTH I/? and GTH II£ I Underyearling h Yearling 2-years-old 200 100 10 12345678 123456789 0.1 12 9101112 12 3 4 5 6 7 8 9101112 10 1988 1989 1990 Fig. 4. Seasonal changes in body weight and gonadosomatic index (GSI) of masu salmon from February 1988 to October1990. Numbersbesideeachsymbolindicatethenumberoffishemployed. Eachvalueisexpressedasthe mean (point) and the standard error (bar). * (p<0.05), ** (p<0.01), and *** (p<0.001) indicate the levels of significant differences. Changes of GnRH in Female Masu Salmon 379 contents are shown in Fig. 5A. Pituitary GTH I/? GTH 11/? showed a gradual increase until July contents showed clear seasonal changes: high in 1990. Thereafter the contents increased rapidly autumn after increases from spring, and low in until October 1990, in accordance with the winter. A distinct decrease was seen in January advancement of vitellogenesis and ovulation. 1989 and a distinct increase was observed during Changes in pituitary GTH I/? and GTH 11/? vitellogenesis and ovulation in 1990. concentrations were similar to those in contents 10000 1000 « 100 10 0.1 4 5 6 7 8 9101112 1 2 3 4 5 6 7 8 9 101112 1 2 3 4 5 6 7 8'9'10' 1988 1989 1990 1000 B — m GTH IB ^-v A o n 1— I 1115 (0 2 ioo- '5. E c X 10- o 4 5 6 7 8 91011121 2 3 4 5 6 7 8 91011121 2 3 4 5 6 7 8 9 10 1988 1989 1990 Fig. 5A and B. Seasonal changes in pituitary GTH I/? (square) and GTH II/3 (triangle) contents (A) and concentrations (B) ofthe masu salmon from April 1988to October 1990and from March 1989 to October 1990, respectively. Presentation ofstatistical data is as in Fig. 4. 380 M. Amano, K. Aida et al. (Fig. 5B). Thereafter, concentrations decreased until September 1989, increased again until January sGnRH in the brain andpituitary 1990, and then decreased until July 1990. Brain sGnRH was already detectable at hatch- sGnRH concentrations in the discrete brain ing, and total brain content increased with growth areas are shown in Figs. 7A-E. They showed a during the underyearling stage (Fig. 6A). There- tendency to decrease from winterthrough summer after, increases were observed from March to May and to increase from autumn through winter. 1989 and from September to January 1990. A sGnRH concentrations in the olfactory bulbs and decrease was observed from May to September telencephalon increased significantly during vitel- 1989. logenesis andovulation. sGnRHconcentrationsin Changes in brain sGnRH concentrations are the hypothalamus also showed a similar tendency. shown in Fig. 6B. A significant decrease was On the contrary, no significant changes were seen observed from April to August 1988, and a sig- in the cerebellum and medulla oblongata during nificant increase was seen in September 1988. vitellogenesis and ovulation. 23456789 23456789 12 1 2'3'4 5 6 7 8 9101112 1 101112 1 10 1988 1989 1990 30 c 2 20 n c 10 °121 2 3 4 5 6 7 8 91011121 2 3 4 5 6 7 8 91011121 2 3 4 5 6 7 8 9 10 1988 1989 1990 Fig. 6A and B. Seasonal changes in brain sGnRH contents (A) and concentrations (B) of the masu salmon from December 1987 to October 1990 andfrom March 1988 to October 1990, respectively. Presentation ofstatistical data is as in Fig. 4. Fig. 7A-E. Seasonal changes in sGnRH concentrations in olfactory bulbs (A), telencephalon (B), hypothalamus (C), optictectum-thalamus (D) andcerebellum and medullaoblongata (E) ofthe masu salmonfrom September 1988 to October 1990. Presentation ofstatistical data is as in Fig. 4. Changes of GnRH in Female Masu Salmon 381 50 A Olfactory bulbs 40 30 20 10 50 B t\ Telencephalon 40 30 20 10 50- Hypothalamus 40- 30 c 20 I ccc 10 o 0- -i 1 1 1 r t 1 1 1 1 r 40 D Optic tectum-thalamus 30 20 10 10 8 6 Medulla 4 Cerebellum- Medulla V-4^ 2 * --—•- 9 101112 1 2 3 4 5 6 7 8 9101112 1 2 3 4 5 6 78 910 1988 1989 1990 382 M. Amano, K. Aida et al. 200 150 vO> 3 DIO 100 § 3 50 2345678 2345678 4 5 6 78 91011121 91011121 910" 1988 1989 1990 Fig. 8. SeasonalchangesinsGnRHcontentsandconcentrationsinthepituitaryofthemasusalmonfromApril 1988 to October 1990. Presentation ofstatistical data is as in Fig. 4. PituitarysGnRHcontentssignificantlyincreased sGnRH did, especially in optic tectum-thalamus. from July through September 1988, from May However, in contrast to sGnRH, no remarkable through November 1989 and from May through changes were observed during vitellogenesis and October 1990as shown in Fig. 8. PituitarysGnRH ovulation. concentrations also increased significantly from May through November 1989 and from May DISCUSSION through October 1990. This is the first report which shows long term cGnRH-II in the brain andpituitary changes in two types of GnRH, sGnRH and cGnRH-II was undetectable at the first two cGnRH-II, inthebrainandpituitary, andGTHsin samplings (December 1987 and January 1988) and thepituitaryin fish. The observationperiodcovers became detectable from February 1988. Brain nearly the entire life cycle from hatching through cGnRH-II contents increased as fish grew, but ovulation. were lower than those of sGnRH (Fig. 9A). sGnRH was detected in the brain just after Brain cGnRH-II concentrations increased signi- hatching, whereas cGnRH-II was first detected ficantly from August 1988 through January 1989 two months later. It is remarkable that both and from September 1989 through January 1990. GnRHs appear during early developmental stages On the contrary, concentrations decreased from when the ovary was still in an immature stage. January 1989 through September 1989 (Fig. 9B). Both GnRH contents increased with a growth cGnRH-II contents in the olfactory bulbs and during underyearling stage. The time lag between pituitary were below the detectable limit in almost the appearance of sGnRH and cGnRH-II, and all individuals throughout the experiment. subsequent development of a differential distribu- cGnRH-II concentrationsin discrete brain areas tion of sGnRH and cGnRH-II suggest the differ- are shown in Figs. 10A-D. They showed atenden- ence in their physiological function. cyto decreasefrom winterthrough summer and to sGnRH concentrations in the olfactory bulbs increase from autumn through winter as those of and the telencephalon, and pituitary sGnRH con- Changes of GnRH in Female Masu Salmon 383 1.51 A c re ni_ O) c 0.5 121 2 34 5 67 8 9101112 1 23 45 67 8 9 101112 1 2 3 4 5 6 7 8 910 1988 1989 1990 B 4 c *<5 C v 121 2345678 91011121 2345678 91011121 234567 8 9 10 1988 1989 1990 Fig. 9A andB. Seasonalchangesin brain cGnRH-II contents (A) and concentrations (B) ofthe masu salmon from December 1987 to October 1990 and from March 1988 to October 1990, respectively. Presentation ofstatistical data is as in Fig. 4. tents increased significantly during vitellogenesis through the regulation of GTH synthesis and re- and ovulation (Figs. 7, 8). Moreover, sGnRH lease. concentrations in the hypothalamus showed a GTH I is considered to regulate the early stages tendency to increase. These changes may be of gonadal maturation, and GTH II is considered related to gonadal maturation, since pituitary mainly to regulate ovulation and spermiation [9]. GTH I/? and GTH 11/? contents and GSI also Pituitary GTH I/? contents showed clear seasonal increased significantly in this period (Figs. 4, 7). changes: contentsincreasedfromspringtoautumn On the other hand, no significant changes in and decreased in winter (Fig. 5A). Pituitary cGnRH-II concentration in the discrete brain sGnRH contents showed stepwise increases from areas were observed (Fig. 10). These results sug- spring to autumn for three years (Fig. 8). Such gest that sGnRH participates mainly in vitel- increases in pituitary GTH I/?contents were likely logenesis and ovulation of this species possibly correlated with the increase in pituitary sGnRH 384 M. Amano, K. Aida et al. A Telencephalon n NDND 0^ ' r -i 1 1- t 1 i 1 r- Hypothalamus NDND l 1 i r 3 W A 5 3 c r 2 Optic tectum-thalamus I o -i 1 1 r- -i 1 i 1 1 1 r- 10 8 Medulla 6 4 Cerebellum- 2 Medulla 91011121 2 3 4 5 6 7 8 91011121 2 3 4 5 6 7 8 910 1988 1989 1990 Fig. 10A-D. Seasonal changes in cGnRH-II concentrations in telencephalon (A), hypothalamus (B), optic tectum- thalamus (C) and cerebellum and medulla oblongata (D) ofthe masu salmon from September 1988 to October 1990. Presentation ofstatistical data is an in Fig. 4. contents. Two types of GTH I (stable and un- Changes in pituitary GTH 11/?contents were simi- stable) are known to exist in the chum salmon lar to those of GSI: after gradual increases until pituitary [10]. Since GnRH extraction was under- the second spring (two year-old fish), rapid in- GTH taken under acidic conditions, unstable I is creases occurred in accordance with vitellogenesis considered to have dissociated to its a and /? and ovulation. Changes in GTH 11/? may reflect subunits. Therefore, changes in GTH 1/3 may those of GTH II, since GTH II is considered to reflect those of unstable GTH I. In order to dissociate under the acidic conditions employed in confirm this hypothesis, GTH I should be mea- GnRH extraction. sured by RIA; however, at present, aspecific RIA sGnRH and cGnRH-II concentrations in the for GTH I has not been established in our labora- brain showed a tendency to increase from autumn tory. Since no significant histological changes of through winter and to decrease from winter ovaries were observed when pituitary GTH I/? through summer (Figs. 6B, 7, 10). Since water contents were high in underyearling and yearling temperature was constant throughout the experi- autumn, the complete form of GTH I may not be ment (9-10°C), photoperiod may be involved in produced or secreted during first two years. the regulation of the synthesis and release of
