Carbonyl Compounds We have already seen a number of carbonyl compounds Ketone two R ... PDF
Preview Carbonyl Compounds We have already seen a number of carbonyl compounds Ketone two R ...
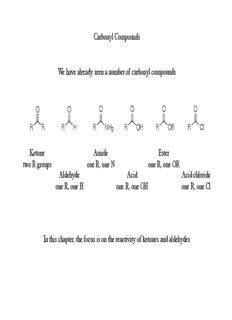
Carbonyl Compounds We have already seen a number of carbonyl compounds O O O O O O R R R H R NH R OH R OR R Cl 2 Ketone Amide Ester two R groups one R, one N one R, one OR Aldehyde Acid Acid chloride one R, one H one R, one OH one R, one Cl In this chapter, the focus is on the reactivity of ketones and aldehydes Nomenclature of Ketones and Aldehydes Many of the same IUPAC rules already learned apply With ketones: suffix is –one With aldehydes: suffix is -al In numbering, the carbonyl takes precedence over previous functional groups learned O O O HO H 2-butanone 4-hydroxy-2-butanone propanal (carbonyl precedence (do not need to write 1) over alcohol) Common Names In addition to the IUPAC naming, many ketones and aldehydes have common names O O O CH 3 H C CH H C H H 3 3 3 acetone methyl ethyl ketone formaldehyde (name two alkyl groups) The aceto- common name is consistent amongst many compounds O O O H C H C OH H C O 3 3 3 aceto- acetic acid ethyl acetate The properties of ketones and aldehydes are dependent on the structure The carbonyl double bond is a result of a π bond between the carbon and oxygen Due to electronegativity of oxygen, this double bond is more polarized than an alkene O O R C O R R R R R The increase in proportion of charged structure in resonance hybrid causes two effects: 1) Carbonyl carbon is more electrophilic (compared to alkene) 2) Oxygen is more nucleophilic (compared to alkene) Can observe differences by looking at electron density maps H H O H H H H Physical properties of ketones and aldehydes Two main physical properties are a direct result of carbonyl functionality: 1) Ketones and aldehydes have a higher boiling point than alkanes of similar mass A result of having a greater charged component in the resonance hybrid (charged structures have a higher boiling point and melting points than uncharged) 2) Ketones and aldehydes have a higher water solubility compared to alkanes H O H Due to hydrogen bonding capability O of carbonyl oxygen R R Synthesis of Ketones and Aldehydes We have already seen many ways to synthesize ketones and aldehydes Other Routes to Ketones and Aldehydes Many carbonyls can be converted to other types of carbonyls (chapter 21 focuses on many of these interconversions) Carboxylic acids can be converted to ketones with organolithium reagents O R Li O OH R Li OH H C OH H C O H C 3 3 3 R First equivalent of organolithium abstracts hydrogen O Second equivalent reacts at carbonyl H C R 3 Upon protonation generate geminal diol Geminal diols equilibrate with ketone Acid Chlorides can be Converted to Either Aldehydes or Ketones The carboxylic acid can first be converted to an acid chloride The acid chloride can be reduced to an aldehyde with lithium tri(t-butoxy) hydride Need to use lithium tri(t-butoxy) hydride since it is weaker than LAH (LAH would reduce aldehyde to alcohol) Acid Chloride to Ketone As seen earlier with most carbon based nucleophiles (e.g. Grignard reagents) two equivalents will react with an acid chloride to yield a tertiary alcohol Dialkyl cuprates (Gilman reagent), however, only one equivalent adds to yield a ketone
Description: