Buchwald-Hartwig C-C Bond Formation PDF
Preview Buchwald-Hartwig C-C Bond Formation
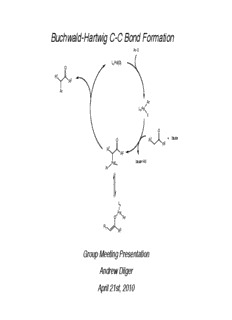
Buchwald-Hartwig C-C Bond Formation Ar-X L Pd(0) n O R1 R2 Ar Ar L Pd n X O O R1 + base R2 R1 R2 PdL base•HX n Ar L n Pd O Ar R 1 R2 Group Meeting Presentation Andrew Dilger April 21st, 2010 3 2 Csp -Csp C-C Bond Formation X Pd cat. unactivated sp3 C-H R-H aryl halide H Pd cat. aryl C-H R-M alkyl metals H Au or Pd R aryl C-H R-X alkyl halide M i. Pd unactivated sp3 C-H R-H aryl metals ii. Pr, Ru, or Cu and O 2 H dehydrogenation Cu, H-acceptor or aryl C-H/sp3 C-H R-H Pd cat., base, air W X Z Z Pd, Ni, or Cu aryl halide R R acidic sp3 C-H W base Z, W = electron withdrawing group Bellina, F.; Rossi, R. Chem. Rev. 2010, 110, 1082. Scope of This Presentation Z W R O O R1 R1 !-arylated nitriles, amides R2(H) OR2(H) lactams, dicarbonyls Ar Ar ketone enolates ester enolates silyl enol ethers silyl ketene acetals aldehydes enol esters ketimines Bellina, F.; Rossi, R. Chem. Rev. 2010, 110, 1082. Buchwald-Hartwig C-C Bond Formation Presentation Outline ! Introduction ! !-Arylated ketones • Discovery and optimization studies • Direct arylations of ketones and enones; including asymmetric variants • Arylation of silyl enol ethers ! !-Arylated aldehydes, esters, and carboxylic acids • Intra- and intermolecular arylation of aldehydes ! Conclusions Relevant review articles: Bellina, F.; Rossi, R. Chem. Rev. 2010, 110, 1082. Burtoloso, A. C. B. Synlett 2009, 320. Culkin, D. A.; Hartwig, J. F. Acc. Chem. Res. 2003, 36, 234. Lloyd-Jones, G. C. Angew. Chem. Int. Ed. 2002, 41, 953. Synthetic Interest in !-Arylated Carbonyls ! Natural products and pharmaceuticals O O R1 O HO N O OMe N O R R2 O Ar Ph OMe O NH R = H = deoxychilenine 2 rocaglaols R = OH = chilenine Trileptal OMe CHO OH N H Me Me Me CO H O O 2 H Me Me nominine Me O NH HO MeO CO2H Me Naproxen polimastamide A Bellina, F.; Rossi, R. Chem. Rev. 2010, 110, 1082. Selected Methods for !-Arylation of Carbonyls ! Stoichiometric, pre-activated aryl and/or carbonyl substrates O O Ph CO2Et Ph3BiCO3 CO2Et 73% TsHN O N THF-Et O, -60 °C Cu 2 Ph Br + then; BF •OEt , 3 2 wet acetone 75% O OTMS NO 2 F CH Cl /DMSO I 2 2 + -40 °C NO 2 89% Aryl bismuth:Abramovitch, R. A.; Barton, D. H. R.; Finet, J.-P. Tetrahedron, 1988, 44, 3039. Aryl copper: Sacks, C. E.; Fuchs, P. L. J. Am. Chem. Soc. 1975, 97, 7372. Diaryl iodonium: Iwama, T.; Birma, V. B.; Kozmin, S. A.; Rawal, V.H; Org. Lett. 1999, 1, 673. Selected Methods for !-Arylation of Carbonyls ! Photoinduced electron transfer 1,4-dicyanonapthalene CH CN:H O, h" 3 2 Pandey, G.; Krishna, A.; Girija, K.; Karthlkeyan, M. Tetrahedron Lett, 1993, 34, 6631. MeO OTBS MeO O 74% ! Acyl anion addition to o-quinone methide Me I OTES Cl OTBS OH Me N-F N 4 Br + Me Mattson, A. E.; Scheidt, K. A. J. Am. Chem. Soc. 2007, 129, 4508. S CH Cl 2 2 Cl O Me 72% ! Ni-catalyzed coupling of enolate with aryl halide O N N 1. LiCPh , THF, -78 °C O 3 O H 2. Ni(COD) , rt O 2 I OMe 28% O O OMe Semmelhack, M. F.; Chong, B. P.; Stauffer, R. D.; Rogerson, T. D.; Chong, A.; Jones, L. D. J. Am. Chem. Soc. 1975, 97, 2507 Transition Metal-Catalyzed !-Arylation of Carbonyls "The observation of phenylacetone as a side product of an aryl halide amination in acetone solvent inspired the development of a practical synthetic method for the !-arylation of a variety of ketones and carboxylic acid derivatives." Culkin, D. A.; Hartwig, J. F. Acc. Chem. Res. 2003, 36, 234. Simultaneous Reports of Practical Pd-Catalyzed !-Arylations ! Buchwald's initial conditions Pd (dba) (1.5 mol%) 2 3 O ligand (3.6 mol%) O Ar-Br + R R' R R' 63-93% yield NaOt-Bu, THF, 70 °C Ar ligand = BINAP or Tol-BINAP Me Me Me O Fe O O Me Me O NC O 76% 93% 75% O t-Bu n-Bu Me O O Ph O O Ph N Me 83% 64% 84% (3% Pd) Palucki, M.; Buchwald, S. L. J. Am. Chem. Soc. 1997, 119, 11108. Simultaneous Reports of Practical Pd-Catalyzed !-Arylations ! Hartwig's initial conditions Pd (dba) (7.5 mol%) 2 3 O DTPF (9.0 mol%) O Ar-Br + R R R' 51-94% yield R' NaOt-Bu or LiHMDS THF, 70 °C Ar O O O Me N Me Me Me 55% (10% Pd) 94% 79% MeO O O P(Tol) 2 DTPF = Fe CN P(Tol) 2 73% (NaOt-Bu) 69% (LiHMDS) Hamann, B. C.; Hartwig, J. F. J. Am. Chem. Soc. 1997, 119, 12382.
Description: