Approved Drug List, December 2017 PDF
Preview Approved Drug List, December 2017
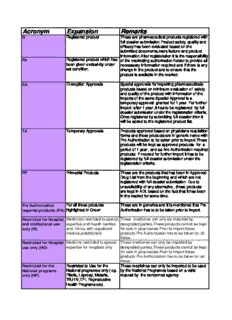
Acronym Expansion Remarks R Registered product These are pharmaceutical products registered with full dossier submission.Product safety, quality and efficacy has been evaluated based on the submitted documents,manufacture and product information.After registeration it is the responsibility Re Registered product which has of the marketting authorization holder to provide all been given exclusivity under neccessary information required and if there is any set condition. change in the product and to ensure that the product is available in the market. EA Exemption Approvals Special approvals for importing pharmaceuticals products based on minimum evaluation of safety and quality of the product with information of the imports of the same.Special Approval is a temporary approval granted for 1 year. For further import after 1 year ,it has to be registered by full dossier submission under the registeration criteria. Once registered by submitting full dossier then it will be added to the registered product list. TA Temporary Approvals Products approved based on physicians requisition forms and these products are in generic name with Pre Authorization to be taken prior to import.These products will be kept as approved products for a period of 1 year , and as Are Authorization required products. If needed for further import it has to be registered by full dossier submission under the registeration criteria. PP Primodial Products These are the products that has been in Approved Drug List from the beginning and which are not registered with full dossier submission. Due to unavailability of any alternative , these products are kept in ADL based on the fact that it has been in the market for some time. Pre Authorization For all those products These are in generics and it is mentioned that Pre required products (PA) highlighted in Green Authorization has to to be taken prior to import. Restricted for Hospital Medicines restricted to special These medicines can only be imported by and institutional use expertise and Health facilties designated parties.These products cannot be kept only (HI) and clinics with registered for sale in pharmacies.Prior to Import these medical practitioners products Pre Authorization has to be taken for all these. Restricted for Hospital Medicine restricted to special These medicines can only be imported by use only (HO) expertise for hospitals only designated parties.These products cannot be kept for sale in pharmacies.Prior to Import these products Pre Authorization has to be taken for all these. Restricted for the Restricted to Use for the These medicines can only be imported to be used National programs National programes only ( eg. by the National Programes based on a valid only (NP) Filaria, Leprosy, Malaria, request by the concerned agency TB,HIV,EPI, Reproductive Health Programs etc) CONTROLLED ( C ) Controlled Drugs which These are medicines which are controlled and can include Narcotics and only be imported by designated parties.From these Psychotropics (Internationally medicines Narcotics cannot be kept in pharmacies and Nationally Controlled). for sale OTC Over the counter medicine These are medicines that can be sold without (OTC) prescriptions POM Prescription only medicine These are medicines that can be sold to a valid (POM) prescription only. E Essential medicine These are medicines that are essential and is in the Essential medicine list Updated on:10th October 2017 Color codes Category Text/Highlighted Colour Explanation Prescription Only Black All the "POM" drugs are in black text Medicine (POM) Over the Counter (OTC) Blue All the "OTC" drugs are in blue text CONTROLLED Purple All the "Controlled" medicine are in purple text Restricted for Hospital Dark Blue All the "Restricted for Hospital Use Only" use only medicines are in dark blue text Restricted and to be Dark Red All the "Restricted and to be used for the used for the National National programme only" medicines are in dark programme only red text Pre-Authorization required before import All the "Pre-Authorization required before import" items are highlighted in light green Products which will be removed soon "Products which will be removed soon" are highlighted in red colour c Maldives Food and Drug Authority Ministry of Health Male’, Republic of Maldives Approved Drug List Number: MTG/RE-AL/Li 0009/2017-0004 o Product Generic name Product name Manufacturer / Dosage form strength Catergory RegisTAa Essen Reg Date Remarks N al No Company name tion tiality eri status S 1 P3639 (1) GLUCOSE 11% 885 ML CONTAIN: Kabiven Peripheral 1000 Fresnius kabi AB, S- Intravenous (1) Glucose 11% 885 ml contain: Restricted for Re 15.08.2017 Can be imported by State GLUCOSE (ANHYDROUS) PH. EUR. 97G, WATER kcal 75174 Uppsala, emulsion for Glucose (anhydrous) Ph. Eur. 97g, Water Hospital use 14.08.2022 Trading Organization Plc FOR INJECTIONS PH. EUR TO 885 ML. Sweden Infusion for injections Ph. Eur to 885 ml. o n l y Each Shipment Only (2) VAMIN ® 18 NOVUM 300ML CONTAIN: (2) Vamin ® 18 Novum 300ml contain: should be ALANINE PH.EUR. 4.8G, ARGININE PH.EUR. Alanine Ph.Eur. 4.8g, Arginine Ph.Eur. accompanied 3.4G, ASPARTIC ACID PH.EUR. 1.0G, GLUTAMIC 3.4g, Aspartic acid Ph.Eur. 1.0g, Glutamic by the batch ACID PH.EUR. 1.7G, GLYCINE PH.EUR. 2.4G, acid Ph.Eur. 1.7g, Glycine Ph.Eur. 2.4g, certificates HISTIDINE PH.EUR. 2.0G, ISOLEUCINE PH.EUR. Histidine Ph.Eur. 2.0g, Isoleucine Ph.Eur. 1.7G, LEUCINE PH.EUR. 2.4G, LYSINE 1.7g, Leucine Ph.Eur. 2.4g, Lysine HYDROCHLORIDE PH.EUR. 2.4G, LYSINE hydrochloride Ph.Eur. 2.4g, Lysine HYDROCHLORIDE PH.EUR. CORRESPONDING TO hydrochloride Ph.Eur. corresponding to LYSINE 2.7G, METHIONINE PH.EUR. 1.7G, Lysine 2.7g, Methionine Ph.Eur. 1.7g, PHENYLALANINE PH. EUR. 2.4G, PROLINE Phenylalanine Ph. Eur. 2.4g, Proline PH.EUR. 2.0G, SERINE PH.EUR. 1.4G, Ph.Eur. 2.0g, Serine Ph.Eur. 1.4g, THREONINE PH.EUR.1.7G, TRYPTOPHAN Threonine Ph.Eur.1.7g, Tryptophan PH.EUR.0.57G, TYROSINE PH.EUR. 0.07G, Ph.Eur.0.57g, Tyrosine Ph.Eur. 0.07g, VALINE PH.EUR. 2.2G. CALCIUM CHLORIDE Valine Ph.Eur. 2.2g. Calcium chloride 2H2O PH.EUR. CORRESPONDING TO CALCIUM 2H2O Ph.Eur. corresponding to Calcium CHLORIDE 0.22G, SODIUM chloride 0.22g, Sodium GLYCEROPHOSPHATE glycerophosphate PH.FRANC.CORRESPONDING TO SODIUM Ph.Franc.corresponding to Sodium GLYCEROPHOSPHATE (ANHYDROUS) 1.5G, glycerophosphate (anhydrous) 1.5g, MAGNESIUM SULPHATE 7H2O PH.EUR. Magnesium sulphate 7H2O Ph.Eur. CORRESPONDING TO MAGNESIUM SULPHATE corresponding to Magnesium sulphate 0.48G, POTASSIUM CHLORIDE PH.EUR. 1.8G, 0.48g, Potassium chloride Ph.Eur. 1.8g, SODIUM ACETATE 3H2O PH.EUR. Sodium acetate 3H2O Ph.Eur. CORRESPONDING TO SODIUM ACETATE 1.5G, corresponding to Sodium acetate 1.5g, WATER FOR INJECTIONS PH.EUR. TO 300ML. Water for injections Ph.Eur. to 300ml. GLACIAL ACETIC ACID PH.EUR. Q.S.TO PH Glacial acetic acid Ph.Eur. q.s.to pH APPROX. 5.6. (3) INTRALIPID ® approx. 5.6. (3) Intralipid 20% 255ML CONTAIN: PURIFIED SOYBEAN OIL ® 20% 255ml contain: Purified soybean PH.EUR. 51G, PURIFIED EGG PHOSPHOLIPIDS oil Ph.Eur. 51g, Purified egg 3.1G, GLYCEROL (ANHYDROUS) PH.EUR. 5.6G, phospholipids 3.1g, Glycerol (anhydrous) WATER FOR INJECTIONS PHEUR TO 255ML PhEur 56g Water for Injections PhEur 2 P3643 (1) GLUCOSE 19% 1053 ML CONTAINS: Kabiven 1900 kcal Fresnius kabi AB, S- Intravenous (1) Glucose 19% 1053 ml contains: Restricted for Re 15.08.2017 - Can be imported by State GLUCOSE (ANHYDROUS) PH. EUR. 200G, WATER 75174 Uppsala, emulsion for Glucose (anhydrous) Ph. Eur. 200g, Hospital use 14.08.2022 Trading Organization Plc FOR INJECTIONS PH. EUR TO 1053 ML. Sweden Infusion Water for injections Ph. Eur to 1053 ml. only Each Shipment Only (2) VAMIN ® 18 NOVUM 600ML CONTAINS: (2) Vamin ® 18 Novum 600ml contains: should be ALANINE PH.EUR. 9.6G, ARGININE PH.EUR. Alanine Ph.Eur. 9.6g, Arginine Ph.Eur. accompanied 6.8G, ASPARTIC ACID PH.EUR. 2.0G, GLUTAMIC 6.8g, Aspartic acid Ph.Eur. 2.0g, Glutamic by the batch ACID PH.EUR. 3.4G, GLYCINE PH.EUR. 4.7G, acid Ph.Eur. 3.4g, Glycine Ph.Eur. 4.7g, certificates HISTIDINE PH.EUR. 4.1G, ISOLEUCINE PH.EUR. Histidine Ph.Eur. 4.1g, Isoleucine Ph.Eur. 3.4G, LEUCINE PH.EUR. 4.7G, LYSINE 3.4g, Leucine Ph.Eur. 4.7g, Lysine HYDROCHLORIDE PH.EUR. CORRESPONDING TO hydrochloride Ph.Eur. corresponding to LYSINE 5.4G, METHIONINE PH.EUR. 3.4G, Lysine 5.4g, Methionine Ph.Eur. 3.4g, PHENYLALANINE PH. EUR. 4.7G, PROLINE Phenylalanine Ph. Eur. 4.7g, Proline PH.EUR. 4.1G, SERINE PH.EUR. 2.7G, Ph.Eur. 4.1g, Serine Ph.Eur. 2.7g, THREONINE PH.EUR.3.4G, TRYPTOPHAN Threonine Ph.Eur.3.4g, Tryptophan PH.EUR.1.1G, TYROSINE PH.EUR. 0.14G, VALINE Ph.Eur.1.1g, Tyrosine Ph.Eur. 0.14g, PH.EUR. 4.4G. CALCIUM CHLORIDE 2H2O Valine Ph.Eur. 4.4g. Calcium chloride PH.EUR. CORRESPONDING TO CALCIUM 2H2O Ph.Eur. corresponding to Calcium CHLORIDE 0.44G, SODIUM chloride 0.44g, Sodium GLYCEROPHOSPHATE glycerophosphate PH.FRANC.CORRESPONDING TO SODIUM Ph.Franc.corresponding to Sodium GLYCEROPHOSPHATE (ANHYDROUS) 3.0G, glycerophosphate (anhydrous) 3.0g, MAGNESIUM SULPHATE 7H2O PH.EUR. Magnesium sulphate 7H2O Ph.Eur. CORRESPONDING TO MAGNESIUM SULPHATE corresponding to Magnesium sulphate 0.96G, POTASSIUM CHLORIDE PH.EUR. 3.6G, 0.96g, Potassium chloride Ph.Eur. 3.6g, SODIUM ACETATE 3H2O PH.EUR. Sodium acetate 3H2O Ph.Eur. CORRESPONDING TO SODIUM ACETATE 2.9G, corresponding to Sodium acetate 2.9g, WATER FOR INJECTIONS PH.EUR. TO 600ML. Water for injections Ph.Eur. to 600ml. GLACIAL ACETIC ACID PH.EUR. Q.S.TO PH Glacial acetic acid Ph.Eur. q.s.to pH APPROX. 5.6. approx. 5.6. (3) INTRALIPID ® 20% 400ML CONTAINS: (3) Intralipid ® 20% 400ml contains: PURIFIED SOYBEAN OIL PH.EUR. 80G, PURIFIED Purified soybean oil Ph.Eur. 80g, Purified EGG PHOSPHOLIPIDS 4.8G, GLYCEROL egg phospholipids 4.8g, Glycerol (ANHYDROUS) PH.EUR. 8.8G, WATER FOR (anhydrous) Ph.Eur. 8.8g, Water for INJECTIONS PHEUR TO 400ML SODIUM Injections PhEur To 400ml Sodium 3 P4004 (1) GLUCOSE 19% 526 ML CONTAINS: Kabiven 900 kcal Fresnius kabi AB, S- Intravenous (1) Glucose 19% 526 ml contains: Restricted for Re 15.08.2017 Can be imported by State GLUCOSE (ANHYDROUS) PH. EUR. 100G, WATER 75174 Uppsala, emulsion for Glucose (anhydrous) Ph. Eur. 100g, Hospital use 14.08.2022 Trading Organization Plc FOR INJECTIONS PH. EUR TO 526 ML. Sweden Infusion Water for injections Ph. Eur to 526 ml. only Each Shipment Only (2) VAMIN ® 18 NOVUM 300ML CONTAINS: (2) Vamin ® 18 Novum 300ml contains: should be ALANINE PH.EUR. 4.8G, ARGININE PH.EUR. Alanine Ph.Eur. 4.8g, Arginine Ph.Eur. accompanied 3.4G, ASPARTIC ACID PH.EUR. 1.0G, GLUTAMIC 3.4g, Aspartic acid Ph.Eur. 1.0g, Glutamic by the batch ACID PH.EUR. 1.7G, GLYCINE PH.EUR. 2.4G, acid Ph.Eur. 1.7g, Glycine Ph.Eur. 2.4g, certificates HISTIDINE PH.EUR. 2.0G, ISOLEUCINE PH.EUR. Histidine Ph.Eur. 2.0g, Isoleucine Ph.Eur. 1.7G, LEUCINE PH.EUR. 2.4G, LYSINE 1.7g, Leucine Ph.Eur. 2.4g, Lysine HYDROCHLORIDE PH.EUR. 2.4G, LYSINE hydrochloride Ph.Eur. 2.4g, Lysine HYDROCHLORIDE PH.EUR. CORRESPONDING TO hydrochloride Ph.Eur. corresponding to LYSINE 2.7G, METHIONINE PH.EUR. 1.7G, Lysine 2.7g, Methionine Ph.Eur. 1.7g, PHENYLALANINE PH. EUR. 2.4G, PROLINE Phenylalanine Ph. Eur. 2.4g, Proline PH.EUR. 2.0G, SERINE PH.EUR. 1.4G, Ph.Eur. 2.0g, Serine Ph.Eur. 1.4g, THREONINE PH.EUR.1.7G, TRYPTOPHAN Threonine Ph.Eur.1.7g, Tryptophan PH.EUR.0.57G, TYROSINE PH.EUR. 0.07G, Ph.Eur.0.57g, Tyrosine Ph.Eur. 0.07g, VALINE PH.EUR. 2.2G. CALCIUM CHLORIDE Valine Ph.Eur. 2.2g. Calcium chloride 2H2O PH.EUR. CORRESPONDING TO CALCIUM 2H2O Ph.Eur. corresponding to Calcium CHLORIDE 0.22G, SODIUM chloride 0.22g, Sodium GLYCEROPHOSPHATE glycerophosphate PH.FRANC.CORRESPONDING TO SODIUM Ph.Franc.corresponding to Sodium GLYCEROPHOSPHATE (ANHYDROUS) 1.5G, glycerophosphate (anhydrous) 1.5g, MAGNESIUM SULPHATE 7H2O PH.EUR. Magnesium sulphate 7H2O Ph.Eur. CORRESPONDING TO MAGNESIUM SULPHATE corresponding to Magnesium sulphate 0.48G, POTASSIUM CHLORIDE PH.EUR. 1.8G, 0.48g, Potassium chloride Ph.Eur. 1.8g, SODIUM ACETATE 3H2O PH.EUR. Sodium acetate 3H2O Ph.Eur. CORRESPONDING TO SODIUM ACETATE 1.5G, corresponding to Sodium acetate 1.5g, WATER FOR INJECTIONS PH.EUR. TO 300ML. Water for injections Ph.Eur. to 300ml. GLACIAL ACETIC ACID PH.EUR. Q.S.TO PH Glacial acetic acid Ph.Eur. q.s.to pH APPROX. 5.6. approx. 5.6. (3) INTRALIPID ® 20% 200ML CONTAINS: (3) Intralipid ® 20% 200ml contains: PURIFIED SOYBEAN OIL PH.EUR. 40G, PURIFIED Purified soybean oil Ph.Eur. 40g, Purified EGG PHOSPHOLIPIDS 2.4G, GLYCEROL egg phospholipids 2.4g, Glycerol (ANHYDROUS) PHEUR 44G WATER FOR (anhydrous) PhEur 44g Water for 4 P4003 (1) GLUCOSE 19% 790 ML CONTAINS: Kabiven 1400 kcal Fresnius kabi AB, S- Intravenous (1) Glucose 19% 790 ml contains: Restricted for Re 15.08.2017 Can be imported by State GLUCOSE (ANHYDROUS) PH. EUR. 150G, WATER 75174 Uppsala, emulsion for Glucose (anhydrous) Ph. Eur. 150g, Hospital use 14.08.2022 Trading Organization Plc FOR INJECTIONS PH. EUR TO 790 ML. Sweden Infusion Water for injections Ph. Eur to 790 ml. only Each Shipment Only (2) VAMIN ® 18 NOVUM 450ML CONTAINS: (2) Vamin ® 18 Novum 450ml contains: should be ALANINE PH.EUR. 7.2G, ARGININE PH.EUR. Alanine Ph.Eur. 7.2g, Arginine Ph.Eur. accompanied 5.1G, ASPARTIC ACID PH.EUR. 1.5G, GLUTAMIC 5.1g, Aspartic acid Ph.Eur. 1.5g, Glutamic by the batch ACID PH.EUR. 2.5G, GLYCINE PH.EUR. 3.6G, acid Ph.Eur. 2.5g, Glycine Ph.Eur. 3.6g, certificates HISTIDINE PH.EUR. 3.1G, ISOLEUCINE PH.EUR. Histidine Ph.Eur. 3.1g, Isoleucine Ph.Eur. 2.5G, LEUCINE PH.EUR. 3.6G, LYSINE 2.5g, Leucine Ph.Eur. 3.6g, Lysine HYDROCHLORIDE PH.EUR. CORRESPONDING TO hydrochloride Ph.Eur. corresponding to LYSINE 4.1G, METHIONINE PH.EUR. 2.5G, Lysine 4.1g, Methionine Ph.Eur. 2.5g, PHENYLALANINE PH. EUR. 3.6G, PROLINE Phenylalanine Ph. Eur. 3.6g, Proline PH.EUR. 3.1G, SERINE PH.EUR. 2.0G, Ph.Eur. 3.1g, Serine Ph.Eur. 2.0g, THREONINE PH.EUR.2.5G, TRYPTOPHAN Threonine Ph.Eur.2.5g, Tryptophan PH.EUR.0.86G, TYROSINE PH.EUR. 0.10G, Ph.Eur.0.86g, Tyrosine Ph.Eur. 0.10g, VALINE PH.EUR. 3.3G. CALCIUM CHLORIDE Valine Ph.Eur. 3.3g. Calcium chloride 2H2O PH.EUR. CORRESPONDING TO CALCIUM 2H2O Ph.Eur. corresponding to Calcium CHLORIDE 0.33G, SODIUM chloride 0.33g, Sodium GLYCEROPHOSPHATE glycerophosphate PH.FRANC.CORRESPONDING TO SODIUM Ph.Franc.corresponding to Sodium GLYCEROPHOSPHATE (ANHYDROUS) 2.3G, glycerophosphate (anhydrous) 2.3g, MAGNESIUM SULPHATE 7H2O PH.EUR. Magnesium sulphate 7H2O Ph.Eur. CORRESPONDING TO MAGNESIUM SULPHATE corresponding to Magnesium sulphate 0.72G, POTASSIUM CHLORIDE PH.EUR. 2.7G, 0.72g, Potassium chloride Ph.Eur. 2.7g, SODIUM ACETATE 3H2O PH.EUR. Sodium acetate 3H2O Ph.Eur. CORRESPONDING TO SODIUM ACETATE 2.2G, corresponding to Sodium acetate 2.2g, WATER FOR INJECTIONS PH.EUR. TO 450ML. Water for injections Ph.Eur. to 450ml. GLACIAL ACETIC ACID PH.EUR. Q.S.TO PH Glacial acetic acid Ph.Eur. q.s.to pH APPROX. 5.6. approx. 5.6. (3) INTRALIPID ® 20% 300ML CONTAINS: (3) Intralipid ® 20% 300ml contains: PURIFIED SOYBEAN OIL PH.EUR. 60G, PURIFIED Purified soybean oil Ph.Eur. 60g, Purified EGG PHOSPHOLIPIDS 3.6G, GLYCEROL egg phospholipids 3.6g, Glycerol (ANHYDROUS) PH.EUR. 6.6G, WATER FOR (anhydrous) Ph.Eur. 6.6g, Water for INJECTIONS PHEUR TO 300ML SODIUM Injections PhEur To 300ml Sodium 5 P3640 (1) GLUCOSE 11% 1180 ML CONTAIN: Kabiven Peripheral 1400 Fresnius kabi AB, S- Intravenous (1) Glucose 11% 1180 ml contain: Restricted for Re 15.08.2017 Can be imported by State GLUCOSE (ANHYDROUS) PH. EUR. 130G, WATER kcal 75174 Uppsala, emulsion for Glucose (anhydrous) Ph. Eur. 130g, Hospital use 14.08.2022 Trading Organization Plc FOR INJECTIONS PH. EUR TO 1180 ML. Sweden Infusion Water for injections Ph. Eur to 1180 ml. o n l y Each Shipment Only (2) VAMIN ® 18 NOVUM 400ML CONTAIN: (2) Vamin ® 18 Novum 400ml contain: should be ALANINE PH.EUR. 6.4G, ARGININE PH.EUR. Alanine Ph.Eur. 6.4g, Arginine Ph.Eur. accompanied 4.5G, ASPARTIC ACID PH.EUR. 1.4.0G, 4.5g, Aspartic acid Ph.Eur. 1.4.0g, by the batch GLUTAMIC ACID PH.EUR. 2.2G, GLYCINE Glutamic acid Ph.Eur. 2.2g, Glycine certificates PH.EUR. 3.2G, HISTIDINE PH.EUR. 2.7G, Ph.Eur. 3.2g, Histidine Ph.Eur. 2.7g, ISOLEUCINE PH.EUR. 2.2G, LEUCINE PH.EUR. Isoleucine Ph.Eur. 2.2g, Leucine Ph.Eur. 3.2G,LYSINE HYDROCHLORIDE PH.EUR. 3.2g,Lysine hydrochloride Ph.Eur. CORRESPONDING TO LYSINE 3.6G, METHIONINE corresponding to Lysine 3.6g, PH.EUR. 2.2G, PHENYLALANINE PH. EUR. 3.2G, Methionine Ph.Eur. 2.2g, Phenylalanine PROLINE PH.EUR. 2.7G, SERINE PH.EUR. 1.8G, Ph. Eur. 3.2g, Proline Ph.Eur. 2.7g, Serine THREONINE PH.EUR.2.2G, TRYPTOPHAN Ph.Eur. 1.8g, Threonine Ph.Eur.2.2g, PH.EUR.0.76G, TYROSINE PH.EUR. 0.09G, Tryptophan Ph.Eur.0.76g, Tyrosine VALINE PH.EUR. 2.9G. CALCIUM CHLORIDE Ph.Eur. 0.09g, Valine Ph.Eur. 2.9g. 2H2O PH.EUR. CORRESPONDING TO CALCIUM Calcium chloride 2H2O Ph.Eur. CHLORIDE 0.30G, SODIUM corresponding to Calcium chloride 0.30g, GLYCEROPHOSPHATE Sodium glycerophosphate PH.FRANC.CORRESPONDING TO SODIUM Ph.Franc.corresponding to Sodium GLYCEROPHOSPHATE (ANHYDROUS) 0.64G, glycerophosphate (anhydrous) 0.64g, MAGNESIUM SULPHATE 7H2O PH.EUR. Magnesium sulphate 7H2O Ph.Eur. CORRESPONDING TO MAGNESIUM SULPHATE corresponding to Magnesium sulphate 0.64G, POTASSIUM CHLORIDE PH.EUR. 2.4G, 0.64g, Potassium chloride Ph.Eur. 2.4g, SODIUM ACETATE 3H2O PH.EUR. Sodium acetate 3H2O Ph.Eur. CORRESPONDING TO SODIUM ACETATE 2.0G, corresponding to Sodium acetate 2.0g, WATER FOR INJECTIONS PH.EUR. TO 400ML. Water for injections Ph.Eur. to 400ml. GLACIAL ACETIC ACID PH.EUR. Q.S.TO PH Glacial acetic acid Ph.Eur. q.s.to pH APPROX. 5.6. (3) approx. 5.6. (3) (3) INTRALIPID ® 20% 340ML CONTAIN: Intralipid ® 20% 340ml contain: SOYBEAN OIL PH.EUR. 68G, PURIFIED EGG soybean oil Ph.Eur. 68g, Purified egg PHOSPHOLIPIDS 4.1G, GLYCEROL (ANHYDROUS) phospholipids 4.1g, Glycerol (anhydrous) PH.EUR. 7.5G, WATER FOR INJECTIONS PH.EUR. Ph.Eur. 7.5g, Water for Injections Ph.Eur. TO 340ML SODIUM HYDROXIDE PH EUR QS To 340ml Sodium hydroxide Ph Eur qs 6 P1306 4-CARBOXYMETHYLAMINO-4-AMINO- Otogesic J&J, India Ear Drops 0.84% + 1.1%(USP) + 0.4% + 76% + POM PP DIPHENYLSULPHONE DIBUCAINE + N.N- 18%(USNF) DIOXYMETHYLCARBOMIDE + GLYCERINE + POLYTHENE GLYCOL (USNF) 7 P369 A and B – Strains ( Propagated on hen’s eggs) VACCINE INFLUENZA Pre-Authorization Injection POM (Controlled) Restricted and TA E 05.04.2017 equivalent to, required before import to be used for - A/Michigan/45/2015 (H1N1) /pdm09 like the National strain (A/Singapore /GP1908/2015,IVR-180 ) programme - A/HongKong/4801/2014 (H3N2) Like strain only (A/HongKong/4801/2014X-263B) - B/Brisbane /60/2008-like strain (B/Brisbane/60/2008, Wild type ) 8 P410 ABACAVIR Abacavir Pre-Authorization Tablet 300 mg Restricted and PP Product registered based required before import to be used for on a special request from a the National clinician programme only 9 P411 ABACAVIR Abacavir Pre-Authorization Oral Liquid 100mg/5ml Restricted and PP Product registered based required before import to be used for on a special request from a the National clinician programme l 10 P2619 ABACAVIR Abacavir Pre-Authorization Oral Liquid 10 mg/ml Restricted and TA 10.07.2012 Product registered based required before import to be used for on a special request from a the National clinician programme only 11 P3793 ABCIXIMAB Abciximab Pre-authorization Injection 10mg/5ml Restricted for TA 11.10.2016- Registered by Physician required before import Hospital use 10.10.2021 Request Form. only 12 P2123 ACARBOSE Glucar - 50 Glenmark , India Tablet 50 mg BP POM R 13 P3045 ACARBOSE (STARCH, MICROCRYSTALLINE Acarbose (starch, Pre-Authorization Tablet 25mg POM PP E 20.05.2014 CELLULOSE, MAGNESIUM STEARATE, AND microcrystalline cellulose, required before import COLLOIDAL SILICON DIOXIDE) magnesium stearate, and colloidal silicon dioxide) 14 P3046 ACARBOSE (STARCH, MICROCRYSTALLINE Acarbose (starch, Pre-Authorization Tablet 100 mg POM PP E 20.05.2014 CELLULOSE, MAGNESIUM STEARATE, AND microcrystalline cellulose, required before import COLLOIDAL SILICON DIOXIDE) magnesium stearate, and colloidal silicon dioxide) 15 P3678 ACECLOFENAC Acelodon Swiss Garnier Life Tablet 100 mg POM Re 01.03.2016- Can be imported by AMDC Sciences, India 28.02.2021 Pvt Ltd Only 16 P2716 ACECLOFENAC Acedol Torrent Tablet 100 mg BP POM R 19.02.2013 Registered by Life Support Pharmaceuticals, India Pvt Ltd 17 P2423 ACECLOFENAC Aceclo Aristo India Tablet 100 mg POM R 25.10.2010 Registered by ADK Company Pvt Ltd 18 P2275 ACECLOFENAC Acenac Medley Ltd, India. Tablet 100 mg (BP) POM R Registered by ADK Company Pvt Ltd 19 P3140 ACECLOFENAC Aeronac Cassel Research Tablet 100 mg (BP) POM R 19.08.2014 My Chemist Laboratories, India 20 P3508 ACECLOFENAC Acenac-SR Medley Ltd, India. Sustained 200 mg BP POM Re 19.05.2015- Can be imported by Adk Release Tablet 18.05.2020 Company Pvt Ltd Only 21 P3267 ACECLOFENAC + LINSEED OIL +METHYL Stednac Stedman Gel 1.5% w/w( BP) + 3% (BP)+ 10%( BP) + 5%( POM R 06.01.2015 AMDC Pvt Ltd SALICYLATE + MENTHOL GEL Pharmaceuticals, India USP) 22 P3389 ACENOCOUMAROL Acenocoumarol Pre-Authorization Tablet 2 mg POM TA 17.02.2015 Product registered based required before import on a special request from a clinician 23 P3390 ACENOCOUMAROL Acenocoumarol Pre-Authorization Tablet 3 mg POM TA 17.02.2015 Product registered based required before import on a special request from a clinician 24 P1764 ACETAZOLAMIDE IP Diamox Wyeth Limited, Plot Uncoated Tablet 250 mg POM PP No. L-137, Phase III-A, Verna Industrial Estate, Verna, Goa 403 722, India 25 P2427 ACETYL SALICYLIC ACID Delisprin 75 Aristo India Tablet (Delayed 75 mg POM R 25.10.2010 ADK Company Pvt Ltd Release) 26 P573 ACETYL SALICYLIC ACID ASA German Remedies Tablet 50 mg POM PP E India 27 P1411 ACETYL SALICYLIC ACID Colsprin Reckkit & Colman, Tablet 325 mg POM PP E India 28 P1412 ACETYL SALICYLIC ACID Colsprin 100 Reckkit & Colman, Tablet 100 mg POM PP E India 29 P1413 ACETYL SALICYLIC ACID ACETYL SALICYLIC ACID Pre-Authorization Tablet 100 mg POM PP E required before import 30 P1414 ACETYL SALICYLIC ACID ACETYL SALICYLIC ACID Pre-Authorization Tablet 300 mg POM PP E required before import 31 P3932 ACETYL SALICYLIC ACID (ASPIRIN) Casprin Y.S.P. Industries (M) Enteric 100 mg POM Re 01.11.2016 - Can be imported by Sdn. Bhd. Lot 3, 5 & 7, Microencaptulat 31.10.2021 Treetop Health Pvt Ltd Jalan P/7, Section 13, ed Capsule only Kawasan Perindustrian Bandar Baru bangi, 43000 Kajang, Selangor Darul Ehsan, Malaysia 32 P122 ACETYLCYSTEINE Mucomix Samarth Life Sciences, Injection 200 mg/ml in 10ml Restricted for EA E 08.10.2017 - India Hospital use 07.10.2018 only 33 P1927 ACETYLSALICYLIC ACID (ASPIRIN) Ecosprin-150 USV Limited, At: 137/B, Enteric coated 150 mg POM PP E New No. 11/3, 4th (gastro-resistant) Main Road, Indl. Town, Tablet Rajajinagar, Bangalore - 560 044, India 34 P1926 ACETYLSALICYLIC ACID (ASPIRIN) Ecosprin-75 USV Private Limited, Enteric coated 75 mg POM PP E INDIA (gastro-resistant) Tablet 35 P1410 ACETYLSALICYLIC ACID IP Disprin Reckkit Benckiser Uncoated 350mg POM PP E (India) Ltd.,At 61 & 62, Effervescent Hootagalli Ind. Area, Tablet Mysore -570 018 India 36 P2611 ACICLOVIR/ ACYCLOVIR Virest Hovid Bhd,Malayisa Cream 5% POM R 10.07.2012 Registered by GKT Pharmacy 37 P2982 ACICLOVIR/ ACYCLOVIR ACYCLOVIR Pre-Authorization Injection 250mg POM PP E 20.05.2014 required before import 38 P2184 ACICLOVIR/ ACYCLOVIR Herperax 800 Micro Labs ltd Tablet 800 mg USP POM R E Registered by ADK Company Pvt Ltd 39 P2185 ACICLOVIR/ ACYCLOVIR Herperax 200 Micro Labs ltd Tablet 200 mg POM R E Registered by ADK Company Pvt Ltd 40 P2607 ACICLOVIR/ ACYCLOVIR Virest Hovid Bhd,Malayisa Tablet 400 mg POM R 10.07.2012 Registered by GKT Pharmacy 41 P2608 ACICLOVIR/ ACYCLOVIR Virest Hovid Bhd,Malayisa Tablet 200 mg POM R 10.07.2012 Registered by GKT Pharmacy 42 P2804 ACICLOVIR/ ACYCLOVIR Zovirax GlaxoSmithKline , Tablet 200 mg POM R 26.08.2013 Registered by ADK Spain Company Pvt Ltd 43 P2805 ACICLOVIR/ ACYCLOVIR Zovirax GlaxoSmithKline , Tablet 800 mg POM R 26.08.2013 Registered by ADK Spain Company Pvt Ltd 44 P2203 ACICLOVIR/ ACYCLOVIR Herperax Micro Labs ltd Ointment 5% POM R E Registered by ADK Company Pvt Ltd
Description: