Alkenes Structure Stability Nomenclature Alkenes PDF
Preview Alkenes Structure Stability Nomenclature Alkenes
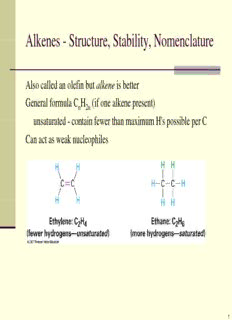
AAllkkeenneess - SSttrruuccttuurree, SSttaabbiilliittyy, NNoommeennccllaattuurree Also called an olefin but alkene is better General formula C H (if one alkene present) n 2n uunnssaattuurraatteedd - ccoonnttaaiinn ffeewweerr tthhaann mmaaxxiimmuumm HH''ss ppoossssiibbllee ppeerr CC Can act as weak nucleophiles 1 SSttrruuccttuurree ooff AAllkkeenneess One C-C sigma bond and one C-C pi bond Presence of C-C pi bond prevents bond rotation! 2 DDeeggrreeee ooff UUnnssaattuurraattiioonn Sum of all multiple bonds and/or rings in a molecule Compared to saturated alkane (C H ) where each double bond n 2n+2 aanndd rriinngg rreemmoovee ttwoo hhyddrrooggeennss. DDooUU == ((HH - HH ))//22 sat actual where H is number of hydrogens in saturated compound sat H is number of hydrogens in compound molecular formula actual andd H = 2 +2 - ##X + ##NN ssaatt nn X = halogens NN == nniittrrooggeennss (ignore other atoms) 3 EExxaammppllee:: CC HH 6 10 H = 2(6) + 2 = 14 (C H ) Sat 6 14 H = 10 actual DoU = 2 Two double bonds? or triple bond? or two rings or ring and double bond 4 EExxaammpplleess C H N C H NBr 7 13 8 8 3 HH = 22((77)) ++ 22 ++ 11 = 1177 HH == 22((88))++22 ++ 11 - 33 == 1166 satt sat H = 13 actual H = 8 actual DoU = 2 DoU = 4 Two double bonds? FFour ddoubblle bbondds?? or triple bond? two triple bonds? or two rings two rings/two or ring and double bond double bonds? 5 AAllkkeennee NNoommeennccllaattuurree Identify longest chain containing the alkene (both carbons) Number carbons in chain so that double bond carbons have lloowweesstt ppoossssiibbllee nnuummbbeerrss ((iiff cchhooiiccee, ggiivvee ssuubb lloowweesstt nnuummbbeerr)) Name, number, and list substituents alphabetically as prefix like alkane nomenclature Indicate position of alkene with number-followed by name of longest chain with suffix -ane replaced with -ene 6 AAllkkeennee NNoommeennccllaattuurree:: IIssoommeerr PPrreeffiixx DDiissuubbssttiittuutteedd AAllkkeenneess:: CCiiss aanndd TTrraannss E and Z System rank two groups on each individual carbon by atomic number if same, find first point of difference count multiple bonds multiple times Highest Priority Groups same side = Z, opposite = E 7 CCyyccllooaallkkeennee NNoommeennccllaattuurree Alkene carbons by definition carbons 1 and 2. Give first substituent lowest possible number. NNaammee, nnuummbbeerr, aanndd lliisstt ssuubbssttiittuueennttss aallpphhaabbeettiiccaallllyy aass pprreeffiixx lliikkee alkane nomenclature You do not need to indicate position of alkene with number, simply name ring size with prefix cyclo and followed suffix -ene For rings smaller the cyclooctene you do not need to include aallkkeennee ggeeoommeettrryy ((oonnllyy cciiss iiss ppoossssiibbllee)) 8 AAllkkeenneess aass SSuubbssttiittuueennttss Methylene VViinnyyll Allyl 9 SSttaabbiilliittyy ooff AAllkkeenneess Evaluate heat given off when C=C is converted to C-C More stable alkene gives off less heat ttrraannss-BBuutteennee ggeenneerraatteess 44 kkJJ lleessss hheeaatt tthhaann cciiss-bbuutteennee 10
Description: