A new genus of groundwater Ameiridae (Copepoda, Harpacticoida) from boreholes in Western Australia and the artificial status of Stygonitocrella Petkovski, 1976 PDF
Preview A new genus of groundwater Ameiridae (Copepoda, Harpacticoida) from boreholes in Western Australia and the artificial status of Stygonitocrella Petkovski, 1976
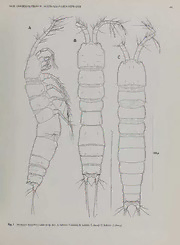
)C^C^^^^'^) Bull. nar. Hist. Mus. Land. (Zool.)68(1): 39-50 Issued27June2002 A new genus of groundwater Ameiridae (Copepoda, Harpacticoida) from boreholes in Western Australia and the artificial status of Stygonitocrella Petkovski, 1976 WONCHOEL LEE DepartmentofLifeSciences, CollegeofNaturalSciences. Hanyang University, Seoul 133-791, Korea: e-mail: @ wlee hanyang.ac.kr RONY HUYS* DepartmentofZoology, TheNaturalHistoryMuseum. CromwellRoad. London SW75BD, U.K.: e-mail: [email protected] SYNOPSIS. Examination of the copepod fauna inhabiting 50m deep production bores on Barrow Island (northwestern Australia), resulted in the discovery of an unusual ameirid which cannot be placed in any extant genus. Both sexes are characterizedbyaunisetoseantennaryexopodandextremereductionintheswimminglegs(particularlytheendopods)andP5. MaleslackadefinedP6closingoffthesinglegenitalapertureandhaveanextraordinarilylargespermatophore.Femalessimilarly displayahighlyreducedgenital field. The newspeciesshowssuperficial similaritiestoboth Psammonitocrella RouchandStygonitocrella Reid, Hunt& Stanley, howeverthecombined presence ofa sexually dimorphic inner basal spine on PI. a completely fused genital double-somite, reduced antennary exopod and vestigial P5 excludes it from either genus. Some problems in the current classification of freshwater Ameiridae are highlighted, with particular reference to the genus Stygonitocrella. A new genus Neonitocrella is proposedforStygonitocrellainsularis(Miura, 1962). INTRODUCTION found yet. The subterranean fauna contains Thermosbaenacea, Syncaridaandadiverseamphipodcommunityincludingbogidiellids and melitids. Our samples were taken in a long abandoned water The arid to semi-arid north-west of Western Australia has a rich production bore (47 m depth) and an abandoned anode bore (55 m stygofaunaincludingtheonlyvertebratetroglobitesknowntooccur depth) that was used in the electrolytic protection ofthe oil wells. in Australasia,the BlindGudgeon,Milyeringa VeritasWhitley,and The latterwould have gassedchlorine and had a pH of<2 when in the Cave Eel. Ophisternon candidum (Mees), and two, supposedly service (Humphreys, pers. comm). sympatric, congeneric shrimps, Stygiocaris lancifera Holthuis and S. stylifera Holthuis with tethyan affinities (Humphreys, 1993). Recently, the freshwater copepods of the Cape Range karst area have been the subject ofintensive study, resulting in thediscovery MATERIALS AND METHODS and description of several cyclopoids belonging to the genera Metacyclops Kiefer, Mesocyclops Sars, Microcyclops Claus, Apocyclops Lindberg,Diacyclops Kieferand Halicyclops Norman Specimensweredissectedinlacticacidandthedissectedpartswere (Pesceetal., \996a-b\ Pesce& DeLaurentiis, 1996; DeLaurentiis mounted on slides in lactophenol mounting medium. Preparations were sealed with Glyceel ortransparent nail varnish. All drawings etai, 1999). have been prepared using a camera lucida on a Zeiss Axioskop Here we reportonthediscovery ofaremarkable harpacticoid in differential interferencecontrast microscope. abandoned production bore holes on Barrow Island off the Cape MalesandfemalesofInermipeshumphreysigenetsp.nov. were Range coast. Barrow Island has a typical island Ghyben-Herzberg examinedwithaPhilipsXL30scanningelectronmicroscope.Speci- systemwithafreshwaterlensoverlyingsaltwater.Thehydrologyof menswerepreparedbydehydrationthroughgradedacetone,critical the superficial karst is little known despite being a production oil point dried, mounted on stubs and sputter-coated with gold or fieldsincetheearly 1960sandbeingthetargetof'producedwater' palladium. dAiussptorsaalliaunatnidl ruepcetnotlayb.ouIttl8ie-s1o0,n0t0h0eyNeoarrtshaWgeositt wSoheullfdohfaWveestbeerenn AbbTrheevidaetsicornisptiuvseedteirnmitnheoltoegxytiasrea:doape,teadesftrhoemtasHcu;ysP1e-tPa6l,. (f1ir9s9t6)t.o part ofthe mainland (and throughout most ofthe last few million sixth thoracopod;exp(enp)-l(2. 3) todenotetheproximal (middle, years) (Humphreys, 2000). The entire island is likely to be an distal) segment of a ramus. Type material is deposited in the anchialinesystembuttodate anentrypointfordivershas notbeen collections ofthe Western Australian Museum, Perth (WAM) and TheNatural History Museum, London (BMNH). *Authorforcorrespondence ©TheNaturalHistoryMuseum,2002 9 40 W. LEEANDR. HUYS SYSTEMATICS 1999.1106),BES792,depth50-55m, 1 December 1992and999, 88in70%alcohol(BMNH 1999.1107-1124),BES793, 1 Decem- WAM ber 1992. Paratypes deposited in are (a) 1 8 dissected on 7 slides, and499, 6 88 in 70% alcohol, BES 792, depth50-55m, 1 FamilyAMEIRIDAE Monard, 1927 December 1992, (b) 1 8 in70% alcohol, BES 798, depth45-50m, GenusINERMIPESgen. nov. 2 December 1992, and (c) 599, 15 88 in 70% alcohol, BES 810, Diagnosis. Ameiridae. Body elongate, cylindrical, and vermi- depth 50-55m, 2 December 1992 (WAM C24415-24417). All specimensarefromthetypelocalityandwerecollectedby DrW.F. form without distinct surface ornamentation except for ventral Humphreys using a plankton net with a 125 m mesh and ofa size spinulepatternsonabdomen.Cephalothoraxandothersomiteswith smoothposteriormargin. Genitalandfirstabdominalsomitescom- suitable forthe borehole (Pesce etal., 1996a). pletely fused forming double-somite; original segmentation not Description discernible. Genital field with small copulatory pore located in Female. Total body length 540-667um (n=10; mean = 592 urn: median depression. Anal operculum welldeveloped. measured from anterior margin ofrostrum to posterior margin of Sexual dimorphism in antennule, PI inner basal spine, P5, P6, caudalrami). Largestwidthmeasuredatposteriormarginofgenital genital segmentation andabdominal spinulation. double-somite: 106pm. Genital double-somiteswollen. Nodistinct Rostrum fusedto cephalothorax, notdefined atbase. Antennule demarcation between urosome andprosome (Fig. IB). 8-segmented in9, 10-segmented and subchirocer in 3; segment 1 Cephalothoraxwithsmoothposteriormargin;pleuralareassmall withoutseta;aesthetascsonsegments4and8(9) oron5and 1 (8); and rounded; sensillae and few pores present as illustrated in Fig. apicalacrothekconsistingofminuteaesthetascand2setae.Antenna 1A-B. Rostrum not defined at base (Fig. IB), with pair of tiny with separate basis and endopod; exopod minute, with 1 seta. sensillae nearapex. Mandible with 2-segmented palp, comprising unarmed basis and Pedigerous somites completely smooth. All prosomites without endopod with 4 setae. Maxillule with strongly developed arthrite, with2nakedsetaonanteriorsurfaceand8spines/setaearounddistal defined hyaline frills; hind margin smooth; separated by large membranous areas. Body not markedly constricted between indi- margin;coxawith2setaeand 1 spine;palpcylindricalwith5 setae, vidualsomites.P4-bearingsomitedistinctlynarrowerthanpreceding probably representing basis only. Maxilla with trisetose endite on ones. syncoxa; allobasis drawn out in remarkably long claw; endopod a Urosome (Fig. 1A-B) 5-segmented, comprising P5-bearing small bisetose segment. Maxilliped with unarmed syncoxa; basis somite, genital double-somite and 3 free abdominal somites. PS- elongate, with very long endopodal pinnate claw. bearing somite, and genital double-somite without surface PI with 3-segmented rami; basis without outerseta, inner spine ornamentation, exceptfordorsal pairsofsensillae. Freeabdominal wseixtuha4lleyldeimmeonrtpsh;iecn;deoxppo-d1 ewliotnhgaftoer,muelxap-[21.w0i.t0h2o0u]t.innerseta,exp-3 somiteswithseveralrowsofminutespinuleslaterally(Fig. 1A)and P2-P4 with 3-segmented exopods and 1-segmented endopods. ventrally (Fig. 2C); hyaline frills ofurosomites not present. Ovary large, about halfofbody length (Fig. 1A-B). Baseswithoutouterseta.Exp-1 markedlyelongate;exp-2withvery Genital double-somite (Figs 1A-B, 2C) wider than long; com- stronginnerseta;apicalsetaeofexp-3verylong.Endopodsminute, pletely fused, with weakly pronounced constriction bilaterally with very long apical seta(e). Armature formula: (possibly indicatingoriginal segmentation). Genital field (Fig. 2C) with small copulatory pore (arrowed in Fig. 2C) locatedin median Exopod Endopod depression; gonopores fused medially forming single genital slit covered on both sides by opercula derived from sixth legs; no P2 0.1.022 010 P3 0.1.022 020 distinct armature discernible; seminal receptacles fused forming P4 0.1.122 010 large median sac. Anal somite (Fig. 2C-D)withwell developed spinulose opercu- P5 rudimentary with baseoendopod fused to somite and repres- lum flanked by spinularrows; withpairofdorsal sensillae anterior einnt$eadnodnl1ymublytioputienrnabtaesaflusseedtas.pEinxeopploudsa1ssmetaallinse8.gmPe6ntruwdiitmhen1tasreyt,a troamoupserwciutlhu7m.setCaaeu;dsaeltaeraIm—iIII(aFingd.V2IC--VIDI)blaroen,gesrettaehaInVwaindde;Vewaicthh formingunarmedmedian operculum in9; asymmetrical in 3 (with minute spinules; setae I andIIpositioneddorsolaterally with setaI dextral or sinistral configuration), represented by opercular un- beingmuchshorterthansetaII;setaIIIpositionedlaterally;setaeIV armedplate. andVfusedbasally,eachwithpredesignedfractureplanesatbase; Caudalramus short, with 7 setae; setaV longest. seta V longest (longer than whole urosomal segments combined); Spermatophore extraordinarily large, longer than halfthe body setaVIsmall; setaVIItri-articulateatbase.Basesofsetaetypically surrounded by few tiny spinules. Innermargin ofeach ramus with length. dorsolateral concavity. TYPEANDONLYSPECIES. Inermipeshumphreysigen. etsp. nov. Antennule (Fig. 3A) 8-segmented, slender; with well developed Etymology. Thegenericnamealludestotheabsenceoftheouter scleritearoundbaseofsegment 1;allsetaebare.Segment 1 without setaonthebases ofthe swimming legs. Gender: feminine. seta; segment 3 longest; segment 4 with aesthetasc. Armature for- mula: l-[0], 2-[7], 3-[7],4-[2 + (1 +ae)], 5-[2], 6-[2], 7-[4], 8-[3+ Inermipes humphreysi sp. nov. acrothek]. Apical acrothek consisting of slender aesthetasc fused basally to 2 setae. All segments without surface ornamentation. Typelocality. Barrow Island, Western Australia (37°5046"N, Antenna(Fig.3B)4-segmented.Coxasmall,withoutornamenta- 31°31'35"W), stn BES 792, borehole, 50-55m deep, 1 December tion. Basis with spinules along proximal outer margin, and distal 1992. innermargin;abexopodalsetaabsent.Exopodminute, 1-segmented, TYPEMATERIAL. Holotype 9dissectedon9slides(WAMC24414). with 1 pinnateapicalseta.Endopod2-segmented;proximalsegment ParatypesdepositedinNHM are 19 dissectedon 8 slides (BMNH with 2 rows of spinules along abexopodal margin; distal segment NEWAMEIRIDAEFROMW.AUSTRALIANGROUNDWATER 41 Fig. 1 Inermipeshumphreysigen.etsp.nov.A,habitus 9,lateral;B,habitus 2,dorsal;C,habitus 6\dorsal. 42 W. LEEANDR. HUYS Fig.2 Inermipeshumphreysigen.etsp.nov.Female:A,maxillule;B,maxilla;C,urosome,ventral [copulatoryporeindicatedbyarrow];D,anal somite andcaudalrami,dorsal;E,P5.Male: F,P5-bearingandgenital somites,ventral [P5aberrantonleftside],ventral;G.P5 [normal];H.P5 [aberrant]. NEWAMEIRIDAEFROMW.AUSTRALIANGROUNDWATER 43 Fig.3 Inermipeshumphreysigen.etsp. nov. Female: A,antennule;B,antenna;C,mandible;D,maxilliped.Male:E,antennule. 44 W. LEEANDR. HUYS longer than proximal; lateral armature arising in proximal half, seta; endopodal lobe not expressed. Exopod forming minute seg- consistingof1 pinnateand2baresetae;apical armatureconsisting ment with 1 bipinnate apical seta. aofnd5sgemnailclutluabteepsoertea;e(s1eegeinnsiecrutl)a;tdeissteatlaefnusdeodpobadsaslelgymteont1pwiintnhatreowseotfa Male. Body larger than in9. Somites bearing P2-P4 wider than spinules along abexopodal margin and 2 transverse hyaline frills in9. Body length 515-690 um (n=20; mean = 622 um; measured fromanteriormarginofrostrumtoposteriormarginofcaudalrami). subapically. Labrum with elaborate spinular ornamentation and pores as in Largest width measuredat P6-bearing somite: 109 urn. Fig. 5A-B. Prosome(Fig. 1C)4-segmented,comprisingcephalothoraxand3 Mandible (Fig. 3C) with well developed gnathobase bearing freepedigeroussomites. Nodistinctdemarcationbetweenurosome severalfine,multicuspidateteetharounddistalmarginand 1 pinnate andprosome.Cephalothoraxwithsmoothposteriormargin;pleural spine atdorsal corner; 1 row ofspinules nearbase ofpalp. Palp 2- areassmallandrounded;sensillaeandfewporespresentasillustrated segmented;proximalsegmentwithoutornamentation;distalsegment inFig. 1C. Rostrumnotdefinedatbase(Fig. 1C), withpairoftiny with4nakedsetaearrangedin2pairs; subapicalpairfusedbasally. sensillae nearapex. Paragnaths (Fig. 5A) strongly developed lobes with medially Pedigerous somites not covered with spinules. All prosomites directed hair-like setules, separated by medial lobe covered with without defined hyaline frills but separated by large membranous dense patternofshort setules. areas. Body without marked constrictions between individual Maxillule (Figs 2A, 5A-B). Praecoxa without ornamentation; somites; P4-bearing somite not distinctly narrowerthanothers. arthriteelongate,stronglydeveloped,with2nakedsetaealongouter Urosome(Fig. 1C)6-segmented. comprisingP5-bearing somite, margin, 2 shortsetaeonanteriorsurface,and6spines/setaearound genital somite and 4 abdominal somites. P5-bearing somite and distalmargin;3distalspinesunipinnateandbearingpore(Fig.5A- genital somite without surface ornamentation, except for pairs of B)atbaseofproximalspinule.Coxawithcylindricalenditebearing dorsal sensillae. Free abdominal somites, including anal somite, 2nakedsetaeand 1 curved,pinnatespine.Palprepresentedbybasis with several rows of spinules laterally and ventrally; dorsal hind andpossiblyincorporatedrami,formingcylindricalsegmentwith5 margin smooth without any ornamentation. Hyaline frills of naked setae arounddistal margin. urosomitesnotpresent. Spermatophoreextraordinarily large,about Maxilla (Fig. 2B) without surface ornamentation on syncoxa; halfofbody length. medial margin membranous and bearing 1 coxal endite with 1 Antennule (Figs 3E, 5C) 10-segmented; haplocer, with pinnatespineand2nakedsetae.Allobasisdrawnoutintoverylong, geniculation between segments 7 and 8. Segment 1 longest, un- slightlycurved,unipinnateclaw;allobasalclawwithspinulesalong armed. Some elements on segments 4, 6 and 8 (inserts in Fig. 3E; distal halfofinnermargin andtransverserow ofposteriorspinules Fig.5C)verysmall.Segment5notswollen.Segment8with 1 small halfwaythelength;accessoryarmatureconsistingof1 pinnatespine fusedspine in mediananteriormargin. Armatureformula: l-[0], 2- onposteriorsurface,and 1 tubeporealongoutermargin.Endopod 1- [7], 3-[6],4-[l + 1 smallspine],5-[4+(1 +ae)],6-[l],7-[l],8-[l + segmented,andlocatedonanteriorsurfaceofallobasis;ornamented 1 fusedspine],9-[4], 10-[5+acrothek]. 1 setaonfifthsegmentvery with 2 naked setae. small [arrowed in Fig. 5C). Apical acrothek consisting ofslender Maxilliped (Fig. 3D) without ornamentation on syncoxa. Basis aesthetasc and 2 naked setae. smoothwithoutornamentation. Endopoddrawnoutintovery long, InnerbasalspineofPI modifiedintorobustbarbedelement(Fig. unipinnate claw without accessory armature; longerthan basis. 4E). P2-P4as in9 SwimminglegsP1-P4(Fig.4A-D)withwideintercoxalsclerites Fifth pair of legs (Figs 2F-H, 5D) fused to supporting somite. andwelldevelopedpraecoxae,both withoutornamentation. Coxae Baseoendopod with short setophore bearing outer basal seta; and bases with anteriorrows ofsurface spinules as figured. Bases endopodal lobe not expressed. Exopod with 2 elements; 1 apical without outer seta. Exopods 3-segmented, endopods 3-segmented tripinnate spinefusedtoexopod,and 1 small slenderbipinnateseta (PI), or 1-segmented (P2-P4). on subapical outer margin (Figs 2G, 5D); abnormal exopod (Fig. PI (Fig. 4A) with large coxa; with 1 rowofspinules on anterior 2H) resembling female condition frequently observed on one or surface. Basiswith 1 strong,bipinnate spine andlong setulesalong eitherside;only 1 outof9malesshowednormalP5exopodonboth inner margin; rows ofspinules along outerdistal margin, and near sides. baseofexopod. Exp-1 and-2with 1 bipinnate slenderspine;exp-3 Sixthpairoflegs(Figs2F,5D)asymmetrical;representedonboth with 1 bipinnate spine, 1 long unipinnate seta and 2 bipinnate sides by small membranous plate (fusedtoventral wall ofsupport- weaklygeniculatesetae.Endopodslightlyshorterthanexopod;enp- ingsomitealongoneside;articulatingatbaseandcoveringgonopore 1 with 1 bipinnate inner seta; enp-2 without seta; enp-3 with 2 alongotherside;dextralandsinistralconfigurationspresent);with- geniculate setae apically. All segments of exopod and endopod outadditional ornamentation. ornamented with small spinules and setules along outer and inner Etymology. The species is named in honour of Dr William F margin asfigured. Humphreys(WesternAustralianMuseum,Perth),whocollectedthe P2-P4 (Fig. 4B-D). Coxae without ornamentation; basis with material. rowofspinulesalongoutermargin;additionalspinules alonginner margin,andonanteriorsurfaceofP3-P4;allsegmentswithpattern of spinules as figured; inner and outer margins of exopod and DISCUSSION endopod segments with setules or spinules. P2 exp-1 longer than exp-2 and -3 combined; P3 exp-1 subequal to exp-2 and -3 com- bined; P4 exp-1 shorter than exp-2 and -3 combined. P2-P4 with Inermipesgennov. isplacedinthefamilyAmeiridaeonaccountof strong innersetaonenp-2, outerexopodal spines slender, terminal themorphologyoftheantennules,mouthpartsandthesexualdimor- setae very long. Endopods minute, represented by small segment. phism in the inner basal spine of leg 1. Within the group of Spine andsetalformulaeas ingeneric diagnosis. freshwater ameirids it can be readily identified by the unisetose Fifth pair of legs (Fig. 2C, E) fused to supporting somite. antennary exopod, the lack of the outer basal seta on P1-P4 the Baseoendopodrepresentedby small, outersetophorebearingbasal extremereductionintheswimminglegs(particularlytheendopods) NEWAMEIRIDAEFROMW. AUSTRALIANGROUNDWATER 45 Fig.4 Inermipeshumphreysigen.etsp.nov. Female: A,PI;B,P2;C,P3;D,P4.Male:E,coxaandbasisofPI. 46 W. LEEANDR. HUYS Fig.S Inermipeshumphreysigen.etsp.nov.SEMmicrographs.A.oralareashowinglabrum.paragnathandpartofmaxillule; B.oralarea(different angle);C,antennule 6[tinysetaonsegment5arrowed];D.abdomen 6,showingP5 andP6.ventral. Scalebars:5|jm(A-B).2pm(C),20|am(D). NEWAMEIRIDAEFROM W.AUSTRALIANGROUNDWATER 47 and the P5. Males lack a defined P6 closing offthe single genital the wrong genus. For example. Nitocrella petkovskii Pesce, 1980 aperture andhave anextraordinarily large spermatophore. Females and Stygonitocrella colchica (Borutzky & Michailova-Neikova, similarly display a highly reduced genital field. The size of the 1970) are very closely related, differing essentially only in the spermatophore, occupying nearly half ofthe body, is remarkable. expression ofthe segment boundary between P4enp-1 and -2, but Except for representatives of the genus Apodopsyllus Kunz areneverthelessplacedindifferentgenera.Theclosezoogeographical (Paramesochridae)wheresimilarlysizedspermatophoreshavebeen connection between N. petkovskii (NW Iran) and S. colchica (W reported,maleharpacticoidcopepodsproducesmallspermatophores, Georgia) is noteworthy in thiscontext. typically not exceeding the length oftwo body somites. Given the /. humphreysi shows superficial similarities to both Psammoni- highly reduced genital apertures of/. humphreysi it is difficult to tocrella and Stygonitocrella, currently the most advanced genera imagine how the spermatophore can be successfully extruded and withintheAmeiridae.howeverthecombinedpresenceofasexually transferred tothe female. dimorphic inner basal spine on PI, a completely fused genital double-somite,reducedantennaryexopodandvestigialP5excludes Taxonomy offreshwaterAmeiridae itfromeithergenus.ThegenusPsammonitocrellawasproposedby Rouch (1992) to accommodate two interstitial species collected in Theprimarytaxonomic subdivisions infreshwaterAmeiridaehave the hyporheic zone ofan intermittent desert stream in Arizona. Its traditionally been based on swimming leg segmentation (Lang, familial placement has been questioned by Martinez Arbizu & 1965; Petkovski, 1976) and have ignored other, more phylo- Moura (1994) who removed the genus from the Ameiridae and genetically informative characters such as setation patterns and regarded itasthesistergroupoftheParastenocarididae.Thiscourse mouthpart morphology (Galassi et al., 1999). This simplistic ofaction was primarily based on the loss ofthe outer spine ofPI approach has led to: (1) the generally unsatisfactory practice of exp-2 andtheabsenceofasexually dimorphic innerbasal spineon describingnewspeciesvirtuallyexclusivelyonlegcharacterswith- leg 1. The juvenile morphology of the P5 in both sexes and the outconsiderationofcephalicappendages,genital fieldmorphology presenceofseparategenitalandfirstabdominalsomitesintheadult or even female abdominal segmentation; (2) the establishment of femalestronglysuggestapaedomorphicoriginforPsammonitocrella. unnatural genera such as Stygonitocrella, and (3) the blurring of The absence ofsexual dimorphism in the innerbasal spineofleg 1 generic boundaries. Central to this confusion stands the genus shouldbere-evaluatedinthiscontext.Themodificationofthisspine Nitocrella whichhasservedasataxonomicrepositoryforfreshwa- in male Ameiridae appears not until the final moult. Delaying the terAmeiridae since its proposal by Chappuis (1924). Lang (1965) expression ofthis characterbeyond the final moult (post-displace- removed all species displaying 3-segmented P2-P4 endopods to a ment)wouldresultinthesecondarylossofsexualdimorphism.The new genus Parapseudoleptomesochra and created asecond genus, absence of a modified inner basal spine in Psammonitocrella is Pseudoleptomesochrella, to accommodate all Nitocrella species thereforetoberegardedasautapomorphicanditisproposedhereto characterizedby 2-segmentedP2-P4endopodsandthe presenceof remove the genus from its floating status and to reallocate it to the an inner seta on P2-P4 exp-1. Even under its revised taxonomic Ameiridae. conceptthegenusNitocrellacontinuedtoaccumulatealargenumber Similarities betweenlnermipesandPsammonitocrellaare found ofnew species which prompted Petkovski (1976) to subdivide the in the presenceofonly twosetationelementson thedistal endopod genus even further. He suggested to group only species with 2- segment ofleg 1, the absenceofthe outerbasal setain P1-P2, and segmented P2-P4 endopods in Nitocrella s. restr. and to reallocate thereducedP4endopod.Itisconceivablethatthesesharedcharacters all remaining species with alternative endopodal segmentation in aretheproductofconvergencesincebothgeneradiffersignificantly two new genera, Nitocrellopsis (P2-P3 3-segmented. P4 2-seg- in the morphology ofthe antenna, maxilla, maxilliped and swim- mented) and Stygonitocrella (P2-P3 1-2-segmented, P4 ming leg ornamentation. 1-segmented). Petkovski(1976)alsorecognizedthreesubgroupsin The supposedly cosmopolitan genus Stygonitocrella was diag- Nitocrella basedon the numberofarmatureelementson P4exp-3: nosedsolelyonthebasisofthe 1-segmentedP4endopod(Petkovski, the hirta- (3-4 setae/spines), chappuisi- (5 setae/spines) and 1976). Comparison ofthe swimming leg setal formulae ofthe 13 vasconica-groups(6setae/spines). Althoughthesegroupshavemet speciescurrentlyincludedstronglyindicatesthepresenceofseveral with general acceptance, their monophyletic status has never been discrete lineages within this genus, each exhibiting atypical arma- challenged.Furthermore,sincePetkovski(1976)didnotdesignatea ture pattern (Table 1) and a restricted geographical distribution typespeciesforStygonitocrella,norforNitocrellopsis,bothgeneric (Table 2). This subdivision is admittedly based on swimming leg nameswereunavailableuntilrecently.Galassietal. (1999)fixedA'. characters only but we suspect it to be at least partly reinforcedby rouchi Galassi, De Laurentiis & Dole-Olivier, 1999 as the type of mouthpartandantennularycharacterswhentheybecomeavailable. Nitocrellopsis, making the name available with theirauthorship. A Formalrecognitionoftheselineagesasdistinctgeneraisimpossible similar course of action was taken by Reid et al. (in press) who since most descriptions are severely lacking in detail and many of designatedS. montana(Noodt, 1965)asthetypeofStygonitocrella. themarebasedonveryfewspecimensoronesexonly.Forexample, Leg segmentation characters should be used with caution when the description ofP. djirgalanica is completely lacking in illustra- inferringrelationshipsin derivedlineages. Forexample, theevolu- tions (Borutzky, 1978). In addition, the type material ofthe great tionary instability ofendopodal segmentation is illustrated by the majority of its species is no longer extant and additional records genus Psammonitocrella Rouchwhich contains species with 1- (P. have notbeen added. 5.petkovskiidiffersfromitscongeners in the longifurcata)or2-segmentedP2-P3endopods(P.boultoni).Unique absenceoftheinnersetaonP3exp-2,anelementwhichispresentin derived characters such as the loss ofthe outer spine on PI exp-2 allothercongeners.Attemptstotracethesinglefemaleonwhichthis leave no doubt that both Psammonitocrella species shared a com- description wasbasedfailed (Galassi, pers. comm.). mon ancestor, and hence the discrepancy observed in endopodal Aspointedoutby Reidetal. (inpress)thegenericplacementof segmentation has to be interpreted as the result of intrageneric S.orghidani(Petkovski, 1973)isquestionable.Theoriginaldescrip- evolution. Utilizing endopodal segmentation patterns in defining tion is very concise, showing illustrations only of the antenna, generic boundaries is potentially misleading. Overweighing such caudalramiandthe fifth legs. Theexopodalarmatureoftheswim- charactersattheexpenseofotherscanresultinassigningspeciesto minglegsislargelyunknown,apartfromPetkovski'sstatementthat 1 48 W. LEEANDR. HUYS -I- I+ + +P-P- + + +-+ + + +^- + + e« EL, tH/i> &H 1+ + +^- + + + + + + + + ~- + + u1-i £aS-3sO 1+ + +^- + + + + + + + + e>- I I \ c~ + + o- c~ + o- + + c~ + + e-. I I O © © —< e-~ © —• — t>- o. o- © d c-~ O O 3* (NmmcNmcirocirocom mmmrn O O CS — O (N e>- (N m <N c^ — c- o- O O O O O O O © O — O fN CN CN t>- (N (N I C-- Nn^tO't^t't^t't m © I c^- c> CS 0©©OIOc^-OCNCNc«-)C'->© O O — © o- < 0. — rjr^^toc^-^-Tj-Tt'S-'^-m rMOmtN oooooooooo O O OOOOOOOOOO o © I CC—NN (C.NN CCNN —CN rc_v^.. CCCNNNCCCNNMCC©NNCC©NNCOCNNCC~NNCCCNNN C<OCNNNOCCCNNNO———OO©CN O O O O O O O O O O O ©©55©o©S«©W© o o Spp2 CONOcnO°. P—. OOOO°.O—:Ocn HOOr OOOO Mpp(NO(Np— r—. (pNptpSMpNpNpNOpI^jNpNOOpOp ddddc-'ddddddd — do'o'd OO OO OO OOO 200g05—5 2oo2o2dod5o2d5d= ==d5d2o — : NNNN^tNIMNNNMN O — poop pppppppSpCNPCNPP — d~-d—d—d—oc>': —-—dd'—d—d—d_d—d—~—d,d„-d_d— OOOO 0000000 OOOO O(NOcnOmOm c-. Om OcnOmOcnOmOmOcn OmOmOMO(N d d d d d d d d d d d d d d d _;_:_'_:_:od — — — —'— — d © c-: N N N M M MOoCdN MOddCN O(—dCNN Md—OCN'• foco. CO-dM^ OddNONddOOIdd)NONddO((ddNNOM—d pdCCNNodC(NNoCCdMNpCdCNN c- Ot*o o £ -2 1 « i- s U 5 = *• 00-5 2 aSz5s -g£q^ e<*>** .i-3ssu: -sot£:^ -33 ago oS S00 s S * o "a oo CN.-51 a -2 t<j 03 a, a:
