A New Genus And Species Of Inseminating Fish (Teleostei : Characidae : Cheirodontinae : Compsurini) From South America With Uniquely Derived Caudal fin Dermal Papillae PDF
Preview A New Genus And Species Of Inseminating Fish (Teleostei : Characidae : Cheirodontinae : Compsurini) From South America With Uniquely Derived Caudal fin Dermal Papillae
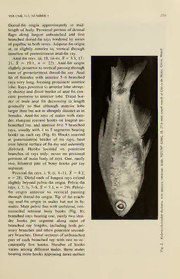
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 13(l):269-283. 2000. 1 A new genus and species of inseminating fish (Teleostei: Characidae: Cheirodontinae: Compsurini) from South America with uniquely derived caudal-fin dermal papillae Luiz R. Malabarba and Stanley H. Weitzman (LRM) Departamento de Zoologia - IB, Universidade Federal do Rio Grande do Sul, Av. Paulo Gama s/n, 90046-900, Porto Alegre, RS, Brasil, and Museu de Ciencias e Tecnologia, PUCRS, Av. Ipiranga 6681, 90619-900, Porto Alegre, RS, Brazil; (SHW) Division of Fishes, Department of Vertebrate Zoology, Smithsonian Institution, Washington, D.C. 20560-0159, U.S.A. — Abstract. Kolpotocheirodon theloura, a new genus and species of the chei- rodontine tribe Compsurini, is described from the uppermost tributaries of the rio Sao Francisco and rio Parana in central Brazil. Males of the new species bear a caudal-fin organ formed by fin-ray hooks and hypertrophied elongate dermal flaps along the fin rays, mostly in the ventral lobe of the caudal fin. The largest flap, attached dorsally to the nineteenth principal caudal-fin ray (ventral principal fin ray of the ventral caudal-fin lobe), is bordered with a series of tabs, and extends dorsally over most of the flaps extending from caudal-fin rays 17 and 18. There are also a series of fleshy papillae distributed along the border of the remaining ventral caudal-fin ray flaps or along the fin rays just dorsal to the flap-bearing fin rays. These tabs and papillae are un- known in both other inseminating and all externally fertilizing characids and therefore are considered derived. The new species is referred to the Compsurini of the Cheirodontinae on the basis of a cladistic diagnosis of these taxa. The relationships of K. theloura with other inseminating compsurins bearing mod- ified caudal—fins are discussed. Resumo. Kolpotocheirodon theloura, genero e especie novos da tribo Compsurini de Cheirodontinae, e descrito para os tributarios superiores do rio Sao Francisco e rio Parana no Planalto Central do Brasil. Machos da nova especie possuem um orgao formado por ganchos nos raios da nadadeira caudal e por dobras de pele ("flaps") hipertrofiadas ao longo dos raios, principalmente O no lobo ventral da nadadeira caudal. maior "flap", conectado ao longo da face dorsal do 19° raio da nadadeira caudal (raio principal inferior do lobo ventral da nadadeira caudal), e marginado por uma serie de tabiques, e se estende dorsalmente sobre as dobras de pele originadas nos 17° e 18° raios. Existe ainda uma serie de papilas carnosas distribuidas ao longo das demais dobras de pele do lobo ventral da nadadeira caudal ou ao longo dos raios imediatamente superiores aos raios com dobras de pele. Estes tabiques e papilas sao desconhecidos em outros caracideos, tanto naqueles com inseminacao como nos com fertilizacao externa, sendo considerados como caracteres derivados. A especie nova e referida aos Compsurini em Cheirodontinae com base em uma diagnose cladistica destes taxons. Sao discutidas as relacoes de K. theloura com outros compsurineos de nadadeiras caudais modificadas. The new genus and species herein de- with modified, possibly glandular tissues, scribed (see Figs. 1-3) is a cheirodontine and hooks on the caudal fin of males. It was 270 PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON discovered in the collections of the Museu is the only species of the Compsurini de Zoologia, Universidade de Sao Paulo by known to have aquasperm (a nearly spher- one of us (L.R.M.) while reviewing chei- ical or spherical sperm nucleus, similar to rodontine characids of southeastern and that found in externally fertilizing chara- eastern Brazil. The species was first hy- cids; see Burns et al. 1997:434, fig. 1A). pothesized to belong to a cheirodontine All other species of the Compsurini so far clade diagnosed by specialized modified investigated have elongate sperm cell bod- anal-fin hooks and derived scales, fin rays ies (see Burns et al. 1997:434, fig. 1B-H). and/or hypertrophied soft tissues on the caudal fin. In the unpublished thesis ofMal- Methods abarba (1994), this clade includes Sacco- The systematic methods, counts and derma Schultz, Compsura Eigenmann, Ma- measurements used here are the same of cropsobrycon Eigenmann, Acinocheirodon & & those described and used by Malabarba Malabarba Weitzman, and the Central " Weitzman (1999). The following acronyms American Odontostilbe" dialeptura (Fink & Weitzman) and "0." mitoptera (Fink & are used for institutions and collections: MCP, Museu de Ciencias e Tecnologia, Weitzman). Later, the new taxon (listed as A Pontificia Universidade Catolica do Rio undescribed genus and species in Burns Grande do Sul, Porto Alegre; and MZUSP, et al. 1997) and all cheirodontine taxa listed Museu de Zoologia da Universidade de Sao above (see Burns et al. 1997) were found Paulo, Sao Paulo. The following abbrevia- to be inseminating species. Subsequently, tions are used in the text or figures: SL = this putative clade was recognized as a standard length; HL = head length; c&s = cheirodontine tribe, the Compsurini (Mala- & alizarin red s and alcian blue stained spec- barba, Weitzman, Burns in Malabarba imens cleared with trypsin; spm(s) = spec- 1998), including the taxon described herein imen^). The comparative material is the (therein referred as new genus and species & same listed in Malabarba Weitzman A). The significance of these characters in (1999). resolving the phylogeny of compsurin chei- rodontines, as well as its possible homology Kolpotocheirodon, new genus to similar characters found in glandulocau- — dine characids, were further discussed by Type species. Kolpotocheirodon thel- & Malabarba Weitzman (1999). oura, new species. — Males of the new genus and species have Diagnosis. The following apomorphies hooks on the caudal fin (Fig. 4) and hyper- diagnose Kolpotocheirodon: A trophied dermal flaps along the caudal-fin specialized caudal organ (Fig. 5) is rays (Fig. 5). The largest flap attached dor- present at the proximal region of the ventral sally to the 19th ventral lobe caudal-fin ray caudal-fin lobe of the males (=character is bordered with a series of tabs, and ex- number 36 in Malabarba 1998). This con- tends dorsally over most of the flaps ex- sists of hypertrophied elongate dermal flaps tending from caudal-fin rays 17 and 18. attached along the fin rays and a series of There is also a series of fleshy papillae dis- relatively flat tabs attached along the ex- tributed along the border of the remaining posed border of the largest flap. The base ventral caudal-fin ray flaps or along the fin of this flap is attached along the anterior rays just dorsal to the fin rays bearing flaps. approximately one-third of the nineteenth These tabs and papillae are unknown in the caudal-fin ray. Visually moving dorsally, remaining inseminating cheirodontines, as the flaps decrease in length and width until well as on other inseminating or externally those of the thirteenth or fourteenth fin rays fertilizing species of characids and are con- are relatively short, narrow, and almost un- sequently hypothesized to be derived. This detectable. Each flap, other than that of the VOLUME NUMBER 113, 271 1 nineteenth ray, bears papillae in a single se- referral of this new genus to the Cheiro- ries along its exposed border. The tabs of dontinae. the nineteenth fin ray flap extend dorsally while the papillae of the fourteenth to at Kolpotocheirodon theloura, new species least the sixteenth or seventeenth flap are Figs. 1-3 directed ventrally. Hooks on the anal-fin rays of mature All specimens from Brazil, Brasilia, Dis- males (Fig. 6) occur along the posteriola- trito Federal, except when noted. MZUSP teral border of the posterior unbranched and Holotype.— 55194, 1 male, 27.3 mm five anterior branched anal-fin rays (Char- SL, small marsh at Curva da Morte, acter 30 in Malabarba 1998). Although the Goias, 3 May—1978, E. K. Bastos. MCP number of hook-bearing anal-fin rays in any Paratypes. Rio Parana tributaries: mm MZUSP species of the Characidae with hooks is var- 11161, 1 male, 25.9 SL, c&s, mm iable according to the degree of maturation, 38840, 2 females, 27.9 and 29.7 SL, MZUSP mm it seems to be constant in fully mature spec- and 38839, 1 male, 29.9 SL, imens of some species. All fully mature ribeirao do Gama, just above the mouth of males of Kolpotocheirodon theloura have ribeirao Taquara, 1 Oct 1985, J. P. Viana MZUSP fully developed hooks restricted to six of Mendes. 39014, 1 female, 24.0 the anterior anal-fin rays. The remaining mm SL and MCP 11160, 1 female, 22.2 mm species of the Compsurini have hooks on a SL, ribeirao Riacho Fundo, tributary of larger number of anal-fin rays, with the ex- rio Sao Bartolomeu, 3 Jul 1985, M. Ribeiro. MZUSP mm ception of the species of Saccoderma, 42802, 1 male, 27.8 SL, 1 fe- mm which have anal-fin hooks only along the male, 24.6 SL, collected with the ho- MCP posterior ray of the anterior unbranched lotype. Rio Sao Francisco tributaries: rays and the four most anterior branched 12204, 4 spms. (1 alcohol, 3 c&s), 14.0- mm MZUSP anal-fin rays. 16.0 SL, and 35722, 12 spms, mm The twelfth and thirteenth caudal-fin rays 14.7-19.5 SL, corrego Vargem de Tras, are somewhat curved, being noticeably 1-2 Apr 1979, N. A. Menezes & E. K. Bas- MZUSP concave along their dorsal borders at about tos. 42801, 2 males, 22.9-23.8 mm mm their basal half lengths (Fig. 4) and some- SL and 1 female, 20.2 SL, lagoa what convex along their ventral borders Feia, 3 May 1978, E. K. Bastos. — where the segments are slightly expanded Diagnosis. The same as for the genus. — longitudinally (character 34, state 2 in Mal- Distinguishing characters. The pres- abarba 1998). This feature separates K. ence of spherical sperm nuclei (aquasperm; theloura from other cheirodontines. Acin- see Burns et al. 1997:434, fig. 1A and tab. & ocheirodon melanogramma Malabarba 1, "undescribed genus and species A") is Weitzman (1999), another compsurin, also plesiomorphic for the Compsurini, accord- has the basal halves of the caudal-fin rays ing to the parsimony analysis in Malabarba dorsally concave with ventrally expanded (1998). Its presence in K. theloura distin- segments, but in this species they are thir- guishes this species from all other species teenth and fourteenth, rather than twelfth of the Compsurini so far investigated, and thirteenth rays. which have elongated sperm nuclei (see — Etymology. The first component of the Burns et al. 1997:434, fig. 1B-H and tab. name Kolpotocheirodon is from the Greek, 1). kolpotos = formed into folds, and refers to Kolpotocheirodon theloura also has an the caudal organ formed by hypertrophied atypical color pattern for the Cheirodonti- dermal folds along the caudal-fin rays. The nae of three to five very small vertical bars second component refers to the characid ge- on the sides of the body, crossing the nar- nus Cheirodon Girard, in reference to our row lateral horizontal body stripe. These are 272 PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON Fig. 1. Kolpotocheirodon theloura, new species, holotype, male, MZUSP 55194, SL 27.3 mm; small marsh at Curva da Morte, Goias, Brazil. located dorsal to the visceral cavity, and be- base of males moderately concave in ante- tween the pseudotympanum and the area rior half and convex posteriorly. In female ventral to the dorsal fin (Figs. 1-3). entire fin base relatively straight. Dorsal Kolpotocheirodon theloura can also be and ventral profile of caudal peduncle con- distinguished from other cheirodontines by cave. Largest mature male examined with a conspicuous dark brown band formed of an expanded and rounded dorsal and ventral a series of spots distributed along all of the caudal-peduncle profiles. Caudal peduncle hook-bearing portion of the anal-fin of the about as long as deep or somewhat shorter males. These pigment spots are placed at than deep. approximately the midlength of the poste- Head small and snout short, rounded. riormost of the anterior unbranched anal-fin Mouth terminal. Maxilla short, positioned rays and the five anteriormost branched at an angle of approximately 45 degrees rel- anal-fin rays. The most conspicuous and ative to long axis of body. Posterior extrem- darkest spot is at the anterior surface of the ity of maxilla reaching vertical that passes first branched ray (Fig. 1; although also pre- through anterior border of eye. sent in the paratypes, the dark brown spot Premaxilla with 4 (rarely 5) symmetrical cannot be seen in the black ground Figs. 2- teeth having 9-11 small evenly spaced A 3). similar, but less conspicuous band of cusps all about equal in size (Fig. 7). Cut- spots occurs in females along the midlength ting edge arched in most medial tooth and of the anal-fin rays. These are more strong- almost straight in most lateral tooth. Max- ly pigmented in the anterior portion of the illa with 2 (rarely 3) teeth, similar in form fin. — to those of premaxilla, with 7-11 cusps. Description. Morphometric data sum- Cutting edge slightly arched to almost marized in Table 1. straight. Dentary with 4 large teeth with 5, Body moderately elongate and com- 7, or 9 cusps; followed by 1 or 2 smaller pressed, greatest depth at dorsal-fin origin. teeth with 3, 5 or 7 cusps. Teeth following Predorsal profile convex, slightly concave second tooth asymmetrical with most lateral in region of supraoccipital process. Profile cusp situated towards tooth base and most of body from base of posterior dorsal-fin medial cusp more distally located. Cusps ray to origin of adipose fin straight or mod- small and regular and approximately equal erately convex. Ventral profile of body con- in size. Cutting edge slightly arched to al- vex from tip of lower jaw to pelvic-fin or- most straight. igin and moderately concave from there to Dorsal-fin rays, ii, 9, n = 29. First un- anal-fin origin. Body profile along anal-fin branched ray about half length of second. VOLUME 113, NUMBER 273 1 Dorsal-fin origin approximately at mid- length of body. Proximal portion of dermal flaps along largest unbranched and first branched dorsal-fin rays bordered by series of papillae in both sexes. Adipose-fin origin at, or slightly anterior to, vertical through insertion of posteriormost anal-fin ray. Anal-fin rays, iii, 18, (ii-iv, X = 3.3, 17- 21, X = 19.1, n = 22). Anal-fin origin O slightly posterior to vertical passing through base of posteriormost dorsal-fin ray. Anal fin of females with anterior 5-6 branched rays very long, forming prominent anterior lobe. Rays posterior to anterior lobe abrupt- > a U ly shorter and distal border of anal fin con- cave posterior to anterior lobe. Distal bor- der of male anal fin decreasing in length gradually so that although anterior lobe larger than but not as abruptly distinct as in females. Anal-fin rays of males with slen- der, elongate retrorse hooks on longest un- branched ray, and anterior first 5 branched r- rays, usually with 4 to 5 segments bearing hooks on each ray (Fig. 6). Hooks inserted ~ at posterolateral border of fin rays, bent nS, over lateral surface of fin ray and anteriorly directed. Hooks located on posterior D branches of rays only; never on proximal portions of main body of rays. One, rarely — two, bilateral pair of bony hooks per ray E segment. X = Pectoral-fin rays, i, 9, (i, 8-11, 9.2, = n 28). Distal ends of longest rays extend slightly beyond pelvic-fin origin. Pelvic-fin rays, i, 7, (i, 7-8, X = 7.1, n = 29). Pelvic- o<u a. fin origin anterior to vertical passing through dorsal-fin origin. Tip of fin reach- c ing anal-fin origin in males but not in fe- 5" males. Male pelvic fins with unilateral, ven- o tromedial retrorse bony hooks (Fig. 8); <^ branched rays bearing one, rarely two slen- der hooks per segment along most of branched ray lengths, including both pri- mary branches and often posterior second- o ary branches. Distal sections of unbranched 5 part of each branched ray with one to oc- casionally few hooks. Number of hooks varies among different males, those males oh bearing more hooks appearing more mature 274 PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON Fig. 3. Kolpotocheirodon theloura, new species, paratype, female, MZUSP 42802, SL 24.6 mm; small marsh at Curva da Morte, Goias, Brazil. when considering other secondary sexual of third caudal-fin ray in dorsal caudal-fin characters such as caudal-fin papillae. lobe with hypertrophied dermal flaps and Principal caudal-fin rays 10/9, n = 29. external papillae only in males. Males with Proximal portion of thirteenth or fourteenth modified 12th to 14th or 15th principal cau- through nineteenth caudal-fin rays in ven- dal-fin rays; these rays bearing (on each tral caudal fin lobe with hypertrophied der- side) row of 4-6 dorsoanteriorly directed mal flaps. Largest flap extending dorsally hooks along dorsal divisions (Fig. 4). Dor- from nineteenth principal caudal-fin ray sal and ventral procurrent rays equivalent in (Fig. 5). Each flap bordered by one series numbers and structure in both sexes, similar of external papillae, more numerous and to those of most characids. Dorsal procur- more developed in males. Proximal portion rent caudal-fin rays, 13 (9-13, X = 10.8, n — Table 1. Morphometries of Kolpotocheirodon theloura, new species. Standard length is expressed in mm; measurements through head length are percentages of standard length; the last four entries are percentages of head length. Range includes the holotype, MZUSP 55194, and following paratypes MZUSP 38839, MZUSP MZUSP MCP MZUSP MZUSP MCP 42801, 42802, 11161, 38840, 39014, 1 1 160. Male;s Females Holotype n Low High X n Low High X Standard length (mm) 27.3 6 22.9 29.9 26.3 5 22.2 29.7 25.7 Snout to anal-fin origin 62.3 6 60.1 63.9 61.8 5 62.2 66.3 65.2 Snout to dorsal-fin origin 49.5 6 48.9 51.5 50.0 5 48.3 53.0 51.1 Snout to pelvic-fin origin 45.4 5 41.7 46.5 44.9 5 44.1 45.5 44.6 Dorsal-fin base length 11.7 5 11.7 13.1 12.5 5 12.6 14.0 13.3 Anal-fin base length 29.3 5 26.4 29.4 28.6 5 26.2 28.5 27.4 Caudal peduncle length 9.9 5 9.9 12.4 11.0 5 11.0 12.2 11.6 Caudal peduncle depth 14.7 5 12.9 14.7 13.7 5 11.3 13.3 12.2 Depth at dorsal-fin origin 35.5 5 32.4 36.1 34.3 5 30.4 37.6 34.4 Dorsal-fin height 28.2 5 27.0 30.4 28.3 5 25.2 29.3 27.9 Pelvic-fin length 20.9 5 19.4 21.7 20.4 5 15.0 18.6 17.1 Pectoral-fin length 23.8 5 20.5 23.8 21.9 4 19.5 23.3 21.4 Bony head length 26.0 5 24.4 26.8 25.8 5 25.9 27.1 26.5 Snout length 18.3 5 18.3 24.1 22.2 5 21.5 24.7 22.9 Upper jaw length 25.4 4 25.4 29.2 27.3 2 29.7 32.2 31.0 Horizontal eye diameter 35.2 5 32.4 39.7 36.1 5 35.6 38.5 36.8 Least interorbital width 29.6 5 29.6 36.2 31.3 5 29.2 31.2 30.6 1 VOLUME 113, NUMBER 275 1 th -15 Fig. 4. Kolpotocheirodon theloura, new species, paratype, MCP 1 1 161, c&s male, SL 25.9 mm. Caudal-fin rays eleven (1 1th) through fifteen (15th) of the ventral caudal-fin lobe, other lower caudal-fin rays only partially indicated. Figure illustrates the position and number of caudal-fin ray hooks along fin rays twelve to fourteen. Anterior is to left. = 5). Ventral procurrent caudal-fin rays, 9 fin rays otherwise white or pale brownish in = 5). yellow. All other fins hyaline except anal Scales cycloid, moderately large. Lateral fin. Anal fin of males with conspicuous line poring incomplete, 8, (6-10, X = 7.6, dark brown band distributed along all hook- n = 21) anterior perforated scales. Scales bearing portions of fin (approximately mid- in lateral series 33, (31-34, X = 32.8, n = dle length of last unbranched anal-fin ray 19). Scale rows between dorsal-fin origin and five anteriormost branched anal-fin and lateral line 5, (5-6, X — 5.1, n = 22). rays). Pattern most conspicuous at anterior Scale rows between lateral line and pelvic- surface of first branched ray. Less conspic- fin origin 4, (3-4, X = 3.6, n = 22). Pre- uous similar band of spots occurs in fe- dorsal scales, when in regular series 1 males, along midlength of most anterior (10-11, X = 10.8, n = 9). Scale rows branched anal-fin rays.— around caudal peduncle 14 {n = 5). Scale Sexual dimorphism. The females lack sheath on anal-fin base consisting of 3-5 hooks on the pelvic, anal, and caudal fins, scales covering unbranched rays and ante- while the males bear numerous small hooks rior 4-5 branched rays. on the pelvic and anal-fin rays. The male Supraneurals, 5 (3-5, X = 4.3, n = 8). holotype and paratypes bear about 4-6 Precaudal vertebrae, 15 (15-16, X = 15.1, hooks on caudal-fin rays 12-14 with more n = 12). Caudal vertebrae, 17 (17-18, X = of these hooks on ray 12 than on ray 14. 17.5, n = 12). — Both sexes bear dermal tabs and papillae on Color in alcohol. Head and body pale the caudal fin, but the females lack these brownish yellow. Black lateral body stripe papillae in the dorsal caudal-fin lobe al- very narrow, pale on caudal peduncle and though they are present in males. The fe- anterior to dorsal-fin origin (Figs. 1-3). Lat- males have the distal border of the anal fin eral body stripe crossed by 3-5 very small with a distinct anterior lobe that is abruptly vertical bars between pseudotympanum and curtailed at about branched rays 6-7. The area ventral to dorsal fin. Humeral spot ab- male distal anal-fin profile is not lobate. In- sent. Caudal-fin base and posterior termi- stead the fin rays gradually diminish in nation of caudal peduncle bear dark black, length from the anterior to posterior termi- vertically-elongate, lozenge-shaped spot nations of the fin. The pelvic fins are longer that reaches dorsal and ventral margins of in the males (19.4-21.7% of SL) than in caudal peduncle. Caudal spot extends just the females (15.0-18.6% of SL), reaching to most proximal portion of middle caudal- past the anal-fin origin in the males, but not 276 PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON Fig. 5. Kolpotocheirodon theloura, new species, holotype, male, MZUSP 55194, SL 27.3 mm. Proximal portion of the ventral caudal-fin lobe. Figure illustrates the derived hypertrophied flaps bearing tabs and papillae along the caudal-fin rays. White arrow indicates largest flap (If) extending dorsally from the nineteenth principal caudal-fin ray (19th), bordered by one series of flat tabs. All other flaps are bordered by one series of fleshy papillae. females. The relative position of the anal fin the distribution of this species in two of also seems to be sexually dimorphic, with these major basins. So far, this species is the snout to anal-fin origin distance in unknown from the rio Tocantins tributaries, males (60.1-63.9% of SL) shorter than in but samples from that area are too rare to females (62.2-66.3% of SL). However, our assure its abse—nce in that drainage. sample size is small and the examination of Etymology. The name theloura is from larger population samples is necessary to the Greek thele meaning nipple and oura confirm this pattern of difference between meaning tail. The name refers to the pres- males and femal—es. ence of papillae on the ventral lobe of the Distribution. Kolpotocheirodon thel- caudal fin. The name is a noun in apposi- oura has an interesting distribution, along tion. — the uppermost tributaries ofboth the rio Sao Discussion. The relationships of Kol- Francisco and the upper rio Parana basins, potocheirodon theloura to other cheirodon- near Brasilia, in central Brazil. It is found tine characids is supported by the presence close to an area called Aguas Emendadas of all four synapomorphies diagnosing the (joint waters), in allusion to the close prox- Cheirodontinae (Malabarba, 1998). These imity of the head waters of the upper trib- are: The presence of a large, nearly trian- utaries of the rio Sao Francisco, alto rio Pa- gular, hiatus among the muscles covering rana and rio Tocantins. This may explain the anterior chamber of the swimbladder VOLUME 113, NUMBER 277 1 Fig. 6. Kolpotocheirodon theloura, new species, paratype, MCP 11161, c&s male, SL 25.9 mm. Anterior portion of anal fin bearing hooks. Figure shows anal-fin hooks positioned along posterolateral border of anal- fin rays, bent anteriorly over lateral surface of anal-fin ray to which it is attached, and distal tip pointing anteriorly. between the first —and second pleural ribs eral border of the anal-fin rays, but are bent (pseudotympanum Figs. 1-3). This hiatus more or less anteriorly over the lateral sur- is limited dorsally by the lateralis superfi- face of the anal-fin ray to which each is cialis muscle, posteriorly by a naked ante- attached. The distal tip of each anal-fin rior face of the second pleural rib, postero- hook is directed towards the anterior border ventrally by the obliquus inferioris muscle, of the anal-fin ray to which it is attached and antero-ventrally by the obliquus super- (Fig. 6), instead of pointing posteriorly or ioris muscle. See also Malabarba (1998: dorsally as in most other characids that 200-201, figs. 2B and 3A) and Weitzman have anal-fin hooks. Hooks are present on & Malabarba (1999:7 and 16, figs. 5, 6, 16 the dorsal surface of some of the caudal-fin and 17). The humeral spot is absent (Figs. rays and inclined towards the caudal-fin 1-3). The teeth are pedunculated, largely base (Fig. 4). Hooks are distributed along expanded and compressed on their distal the distal lengths of the principal caudal-fin borders (Fig. 7). An unique regular, single rays 1 1 to 14 (Fig. 4). The anal fin is more tooth row is present on the premaxilla. The strongly pigmented along the distal portion teeth of this row are perfectly aligned and of the branched rays (Fig. 1). Kolpotochei- similar in shape and cusp number (Fig. 7). rodon theloura was placed as the most bas- Kolpotocheirodon theloura is included al species (identified as Species A) of the among the members of the cheirodontine Compsurini in the parsimony analysis of tribe Compsurini (Malabarba, Weitzman, & Malabarba (1998). We herein further dis- Burns in Malabarba, 1998) because it has cuss and describe the characters of K. thel- the following synapomorphies of that tribe: oura in order to better hypothesize its re- The species is inseminating. The anal-fin lationships to other compsurin species. hooks are positioned along the posterolat- The presence of insemination, where 278 PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON Fig. 7. Kolpotocheirodon theloura, new species, paratype, MCP 11161, c&s male, SL 25.9 mm. Right maxilla and premaxilla and teeth, internal view. sperm are introduced into the ovary (noted just listed above and the hypothesis that the above), is so far found in the species of the Compsurini is a derived branch arising Glandulocaudinae (Burns et al. 1995), in from externally fertilizing cheirodontines the species of the tribe Compsurini of the (see cladogram, fig. 1 of Malabarba 1998) Cheirodontinae (Burns et al. 1997; Mala- suggests a hypothesis that insemination in & barba, Weitzman, Burns in Malabarba the compsurin cheirodontines arose from 1998), and species of uncertain relation- externally fertilizing ancestral cheirodonti- ships including Monotocheirodon pearsoni ne stock, and that it probably arose inde- Eigenmann in Pearson (1924), Brittan- pendently of the Glandulocaudinae and of ichthys axelrodi Gery (1965), " Cheirodon" the other taxa listed above. At least all chei- & ortegai Vari Gery (1980) and "Brycon- rodontines, including the compsurins, lack & americus" pectinatus Vari Siebert the following synapomorphy of the glan- & (1990), (Weitzman Malabarba 1998, dulocaudines used in part to diagnose that & Burns et al. 1999). The absence of cheiro- subfamily by Weitzman Menezes (1998): dontine synapomorphies among the taxa presence of a sperm storage area in the tes- Fig. 8. Kolpotocheirodon theloura, new species, paratype, MCP 11161, c&s male, SL 25.9 mm. Figure illustrates the pelvic-fin rays and hooks in the left pelvic fin, anterior to left. Ventral view.
