6-membered aromatics as factor Xa inhibitors PDF
Preview 6-membered aromatics as factor Xa inhibitors
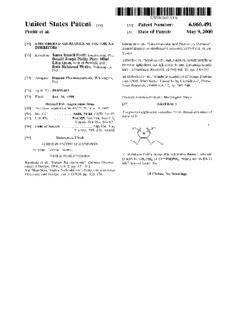
US006060491A United States Patent [19] [11] Patent Number: 6,060,491 Pruitt et al. [45] Date of Patent: May 9, 2000 [54] 6-MEMBERED AROMATICS AS FACTOR XA Edmunds et al., “Cardiovascular and Pulmonary Diseases”, INHIBITORS AnnualReports in Medicinal Chemistry, (1996) vol. 31, pp. 51—60. [75] Inventors: James Russell Pruitt, Landenberg, Pa.; Donald Joseph Phillip Pinto; Mimi TidWell et al., “Strategies for Anticoagulation With Synthetic Lifen Quan, both of Newark, Del.; Protease Inhibitors. Xa Inhibitors Versus Thrombin Inhibi Ruth Richmond WeXler, Wilmington, Del. tors”, Thrombosis Research, (1980) vol. 19, pp. 339—349. [73] Assignee: Dupont Pharmaceuticals, Wilmington, SturZebecher et al., “Synthetic Inhibitors of Serine Protein Del. ases XXIII. Inhibition of Factor Xa by Diamidines”, Th rom bosis Research, (1980) vol. 17, pp. 545—548. [21] Appl. No.2 09/099,663 [22] Filed: Jun. 18, 1998 Primary Examiner—Zinna Northington Davis Related US. Application Data [57] ABSTRACT [60] Provisional application No. 60/050,214, Jun. 19, 1997. The present application describes 6-membered aromatics of [51] Int. Cl.7 ........................ .. A61K 31/44; C07D 211/82 formula I: [52] US. Cl. ........................ .. 514/355; 546/314; 546/315; 564/86; 514/354; 514/631 [58] Field of Search ................................... .. 546/314, 316; 514/354, 355, 631; 564/86 [56] References Cited FOREIGN PATENT DOCUMENTS 9616940 6/1996 WIPO . or pharmaceutically acceptable salt forms thereof, Wherein OTHER PUBLICATIONS D may be CHZNH2 or C(=NH)NH2, Which are useful as Kunitada et al., “Factor Xa Inhibitors”, Current Pharma inhibitors of factor Xa. ceutical Design, 1996, vol. 2, pp. 531—542. Shi—Shan Mao, “Factor Xa Inhibitors”, Perspectives in Drug Discovery and Design, vol. 1 (1993), pp. 423—430. 15 Claims, No Drawings 6,060,491 1 2 6-MEMBERED AROMATICS AS FACTOR XA treatment of thromboembolic disorders. It is thus desirable INHIBITORS to discover neW factor Xa inhibitors. SUMMARY OF THE INVENTION This application claims the bene?t of US. Provisional Application No. 60/050,214, ?led Jun. 19, 1997. Accordingly, one object of the present invention is to provide novel 6-membered aromatics Which are useful as FIELD OF THE INVENTION factor Xa inhibitors or pharmaceutically acceptable salts or This invention relates generally to novel 6-membered prodrugs thereof. aromatics Which are inhibitors of trypsin-like serine protease It is another object of the present invention to provide enzymes, especially factor Xa, pharmaceutical compositions 10 pharmaceutical compositions comprising a pharmaceuti containing the same, and methods of using the same as cally acceptable carrier and a therapeutically effective anticoagulant agents for treatment and prevention of throm amount of at least one of the compounds of the present boembolic disorders. invention or a pharmaceutically acceptable salt or prodrug form thereof. BACKGROUND OF THE INVENTION 15 It is another object of the present invention to provide a WO 96/28427 describes benZamidine anticoagulants of method for treating thromboembolic disorders comprising the formula: administering to a host in need of such treatment a thera peutically effective amount of at least one of the compounds of the present invention or a pharmaceutically acceptable 20 salt or prodrug form thereof. These and other objects, Which Will become apparent during the folloWing detailed description, have been achieved by the inventors’ discovery that compounds of formula (I): 25 30 Wherein Z1 and Z2 are O, N(R), S or OCH2 and the central ring may be phenyl or a variety of heterocycles. The presently claimed compounds do not contain the Z1 linker or or pharmaceutically acceptable salt or prodrug forms the substitution pattern of the above compounds. 35 thereof, Wherein A, B, D, E, M, R“, Rlb, and Z are de?ned WO 95/18111 addresses ?brinogen receptor antagonists, beloW, are effective factor Xa inhibitors. containing basic and acidic termini, of the formula: DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS 40 [1] Thus, in a ?rst embodiment, the present invention provides novel compounds of formula I: Wherein R1 represents the basic termini, U is an alkylene or 45 heteroatom linker, V may be a heterocycle, and the right hand portion of the molecule represents the acidic termini. The presently claimed compounds do not contain the acidic \E /\ termini of WO 95/18111. Activated factor Xa, Whose major practical role is the generation of thrombin by the limited proteolysis of pro or a stereoisomer or pharmaceutically acceptable salt form thrombina holds a central position that links the intrinsic and thereof, Wherein; eXtrinsic activation mechanisms in the ?nal common path ring M contains from 0—4 N atoms; Way of blood coagulation. The generation of thrombin, the ?nal serine protease in the pathWay to generate a ?brin clot, 55 from its precursor is ampli?ed by formation of prothrom binase compleX (factor Xa, factor V, Ca2+ and E is selected from phenyl, 2-pyridyl, 4-pyridyl, pyrimidyl, phospholipid). Since it is calculated that one molecule of and piperidinyl substituted With 1 R; factor Xa can generate 138 molecules of thrombin (Elodi, S., Varadis K.: Optimization of conditions for the catalytic e?rect 60 R is selected from H, F, Cl, Br, I, 0R3, SR3, CO2R3, N02, of the factor IXa-factor VIII Complex." Probable role of the and CH2OR3, and (CR8R9)tNR8R9; complex in the ampli?cation of blood coagulation. Th romb. alternatively, E and R combine to form methylenedioXy or ethylenedioXy; Res. 1979, 15, 617—629), inhibition of factor Xa may be more ef?cient than inactivation of throibin in interrupting th Z is selected from a bond, C1_4 alkylene, (CH2),O(CH2),, e blood coagulation system. (CHZLNRXCHZL, (CHZWOXCHZL, (CHZ).C(O)O Therefore, ef?cacious and speci?c inhibitors of factor Xa (CHZL, (CHZ).OC(O> (0112)., (CHZ>.C(O>NR3(CHZ)., are needed as potentially valuable therapeutic agents for the (CHZ>.NR3C(O>(CHZ),, (CHZ).OC(O)O(CHZ)., (CH2) 6,060,491 3 4 r Oc(O)NR3(cH2>r, (cH2),NR3c(O)O(cH2)r, (CH2), R317, at each occurrence, is selected from H, CL4 alkyl, NR3c(O)NR3(cH2)r, (cH2>rs(O>p(cH2>,, (CH2), and phenyl; SO2NR3(CH2),, (CH2),NR3SO2(CH2),, and (CH2), R36, at each occurrence, is selected from C1_4 alkyl, and phenyl; NR3SO2NR3(CH2)r, provided that Z does not form a N—N, N—O, N—S, NCHZN, NCHZO, or NCHZS A is selected from: bond With ring M or group A; C3_1O carbocyclic residue substituted With 0—2 R4, and R“ and Rlb are independently absent or selected from 5—10 rnernbered heterocyclic system containing from —(CH2),—R1', —CH=CH—R1', NCH2R1", 1—4 heteroatorns selected from the group consisting OCH2R1", SCH2R1", NH(CH2)2(CH2),R1', O(CH2)2 of N, O, and S substituted With 0—2 R4; (CH2)R1V> and S(CH2)2(CH2)R1V; 1O B is selected from: H, Y, and X—Y; alternatively, R1“ and Rlb, When attached to adjacent X is selected from C1_4 alkylene, —CR2 (CRZRZb) (CH2), carbon atoms, together With the atoms to Which they are attached form a 5—8 rnernbered saturated, partially saturated or unsaturated ring substituted With 0—2 R4 and Which contains from 0—2 heteroatorns selected 15 from the group consisting of N, O, and S; alternatively, When Z is C(O)NH and R1“ is attached to a ring carbon adjacent to Z, then R“ is a C(O) Which replaces the amide hydrogen of Z to form a cyclic irnide; R1’ is selected from H, C1_3 alkyl, F, Cl, Br, I, —CN, —CHO, (CF2),CF3, (CH2),OR2, NR2R2“, C(O)R2C, OC(O)R2, (CF2),CO2R2C, S(O)pR2b, NR2(CH2),OR2, Y is selected from: CH(=NR2C)NR2R2“, NR2C(O)R2b, NR2C(O)NHR2b, (CH2),NR2R2“, provided that X—Y do not form a NR2C(O)2R2“, OC(O)NR2“R2b, C(O)NRZRZ“, C(O) 25 N—N, O—N, or S—N bond, C3_1O carbocyclic residue substituted With 0—2 R4“, and NR2(CH2)rOR2, SOZNRZRZ“, NRZSOZRZb, C3_6 car 5—10 rnernbered heterocyclic system containing from bocyclic residue substituted With 0—2 R4, and 5—10 1—4 heteroatorns selected from the group consisting rnernbered heterocyclic system containing from 1—4 of N, O, and S substituted With 0—2 R4“; heteroatorns selected from the group consisting of N, O, and S substituted With 0—2 R4; R1" is selected from H, CH(CH2OR2 2, C(O)R2C, C(O) NR2R2”, S(O)R2b, S(O)2R2b, and SOZNRZRZH; R2, at each occurrence, is selected from H, CF3, CL6 alkyl, benZyl, C3_6 carbocyclic residue substituted With 35 0—2 R417, and 5—6 rnernbered heterocyclic system con taining from 1—4 heteroatorns selected from the group consisting of N, O, and S substituted With 0—2 R4b; R2“, at each occurrence, is selected from H, CF3, CL6 alternatively, one R4 is a 5—6 rnernbered arornatic hetero alkyl, benZyl, phenethyl, C3_6 carbocyclic residue sub cycle containing from 1—4 heteroatorns selected from stituted With 0—2 R417, and 5—6 rnernbered heterocyclic the group consisting of N, O, and S; system containing from 1—4 heteroatorns selected from thebgroup consisting of N, O, and S substituted With 0—2 R4 ; R217, at each occurrence, is selected from CF3, CL4 alkoXy, 45 CL6 alkyl, benZyl, C3_6 carbocyclic residue substituted With 0—2 R417, and 5—6 rnernbered heterocyclic system containing from 1—4 heteroatorns selected from the grclup consisting of N, O, and S substituted With 0—2 R4 ; alternatively, one R4“ is a 5—6 rnernbered arornatic het R26, at each occurrence, is selected from CF3, OH, CL4 erocycle containing from 1—4 heteroatorns selected alkoXy, C1_6 alkyl, benZyl, C3_6 carbocyclic residue from the group consisting of N, O, and S and substi substituted With 0—2 R417, and 5—6 rnernbered hetero tuted With 0—1 R5; cyclic systern containing from 1—4 heteroatorns 55 selected from the group consisting of N, O, and S substituted With 0—2 R4b; alternatively, R2 and R2“, together With the atom to Which they are attached, combine to form a 5 or 6 rnernbered saturated, partially saturated or unsaturated ring sub stituted With 0—2 R417 and containing from 0—1 addi tional heteroatorns selected from the group consisting of N, O, and S; R5, at each occurrence, is selected from CF3, CL6 alkyl, R3, at each occurrence, is selected from H, CL4 alkyl, and phenyl substituted With 0—2 R6, and benZyl substituted phenyl; With 0—2 R6; 65 R3“, at each occurrence, is selected from H, CL4 alkyl, R6, at each occurrence, is selected from H, OH, (CH2), and phenyl; OR2, F, Cl, Br, I, C1_4 alkyl, CN, NO2, (CH2),NR2R2“, 6,060,491 R7, at each occurrence, is selected from H, OH, C1_6 alkyl, 5 CL6 alkylcarbonyl, CL6 alkoXy, CL4 alkoxycarbonyl, (CH2)n-phenyl, C6_1O aryloXy, C6_1O aryloXycarbonyl, C6_1O arylrnethylcarbonyl, CL4 alkylcarbonyloxy C1_4 alkoXycarbonyl, C6_1O arylcarbonyloXy C1_4 alkoxycarbonyl, C1_6 alkylarninocarbonyl, 1O phenylarninocarbonyl, and phenyl-C1_4 alkoXycarbo nyl; R8, at each occurrence, is selected from H, CL6 alkyl and (CH2)n-phenyl; 15 alternatively, R7 and R8 combine to form a 5 or 6 rnernbered saturated, ring Which contains from 0—1 lb additional heteroatorns selected from the group con- R \ —N\ sisting of N, O, and S; 20 \ /N R9, at each occurrence, is selected from H, CL6 alkyl and (CH2)n-Ph@ny1; n is selected from 0, 1, 2, and 3; In is selected from 0, 1, and 2; 25 /—N/Rlb p is selected from 0, 1, and 2; N\ / r is selected from 0, 1, 2, and 3; s is selected from 0, 1, and 2; and, 30 t is selected from 0 and 1. [2] In a preferred embodi.m ent, the present i. nventi. on N=\/\ R1b provides novel compounds of formulae Ia—Io: \ /N 35 21 R13 f/“AR 40 _ _ 1b N N \ / b 45 R13 /__ _—\ /R 1b N=N Rlb 50 55 / 65 6,060,491 -continued Rlb _N 11% 1O and Rlb N/ N 15 as wherein: Z is selected from a bond, CHZO, OCH2, CHZNH, NHCH2, CH2C(O), C(O)CH2, C(O)NH, C(O)NH, CH2S(O)2, S(O)2(CH2), SOZNH, and SOZNH; 25 B is selected from: Y, X—Y, and NRZRZH; Y is selected from one of the following carbocyclic and heterocyclic systems which are substituted with 0—2 R4a; phenyl, piperidinyl, piperaZinyl, pyridyl, pyrimidyl, furanyl, morpholinyl, thiophenyl, pyrrolyl, pyrrolidinyl, oXaZolyl, isoXaZolyl, thiaZolyl, isothiaZolyl, pyraZolyl, imidaZolyl, oXadiaZole, thiadiaZole, triaZole, 1,2,3-oXadiaZole, 1,2,4 oXadiaZole, 1,2,5-oXadiaZole, 1,3,4-oXadiaZole, 1,2, 35 3-thiadiaZole, 1,2,4-thiadiaZole, 1,2,5-thiadiaZole, 1,3,4-thiadiaZole, 1,2,3-triaZole, 1,2,4-triaZole, 1,2, 5-triaZole, 1,3,4-triaZole, benZofuran, benZothiofuran, indole, benZimidaZole, benZoXaZole, benZthiaZole, indaZole, benZisoXaZole, benZisothiaZole, and isoindaZole; Y may also be selected from the following bicyclic heteroaryl ring systems: 45 55 N wherein 18y XAN N\ \ K D is selected from C(=NR7)NR8R9 and; (CR8R9)t N’ || u > NR8R9; \/K1\/N and N / /\R4; R is selected from H, F, Cl, 0R3, CH2OR3, CHZNHZ; A is selected from: piperidinyl, K is selected from O, S, NH, and N. 65 piperaZinyl, [3] In a more preferred embodiment, the present invention provides novel compounds of formulae: C5_6 carbocyclic residue substituted with 0—2 R4, and 6,060,491 9 5—6 membered heteroaryl containing from 1—4 heteroa toms selected from the group consisting of N, O, and S substituted With 0—2 R4; Y is selected from one of the following carbocyclic and heterocyclic systems Which are substituted With 0—2 R4a; phenyl, piperidinyl, piperaZinyl, pyridyl, pyrimidyl, furanyl, morpholinyl, thiophenyl, pyrrolyl, pyrrolidinyl, oXaZolyl, isoXaZolyl, thiaZolyl, isothiaZolyl, pyraZolyl, imidaZolyl, benZimidaZolyl, 10 oXadiaZole, thiadiaZole, triaZole, 1,2,3-oXadiaZole, 1,2,4-oXadiaZole, 1,2,5-oXadiaZole, 1,3,4 oXadiaZole, 1,2,3-thiadiaZole, 1,2,4-thiadiaZole, 1,2, 5-thiadiaZole, 1,3,4-thiadiaZole, 1,2,3-triaZole, 1,2,4 triaZole, 1,2,5-triaZole, and 1,3,4-triaZole. [4] In an even more preferred embodiment, the present [5] In a further preferred embodiment, the present inven invention provides novel compounds Wherein: tion provides novel compounds selected from: E is phenyl; N-(2‘-Aminosulfonyl-[ 1, 1‘]biphen-4-yl)-2-(3‘ D is selected from C(=NH)NH2 and CHZNHZ; amidinophenyl)nicotinamide; R is selected from H, F, Cl, and Br; 20 N-[S-(2-aminosulfonyl)phenylpyrid-2-yl]-2-(3‘ A is selected from: amidinophenyl)nicotinamide; C5_6 carbocyclic residue substituted With 0—2 R4, and N-[S-(2-t-butylaminosulfonyl)phenylpyrid-2-yl]-2-(3‘ 5—6 membered heteroaryl containing from 1—3 heteroa amidinophenyl)nicotinamide; and, toms selected from the group consisting of N, O, and S substituted With 0—2 R4; 25 N-[S-(2-aminosulfonyl)phenylpyrid-2-yl]-2-(3‘ carboXamidophenyl)nicotinamide; Y is selected from one of the folloWing carbocyclic and heterocyclic systems Which are substituted With 0—2 or a pharmaceutically acceptable salt thereof. R4a; In a second embodiment, the present invention provides phenyl, piperidinyl, piperaZinyl, pyridyl, pyrimidyl, novel pharmaceutical compositions, comprising: a pharma furanyl, morpholinyl, thiophenyl, pyrrolyl, ceutically acceptable carrier and a therapeutically effective pyrrolidinyl, oXaZolyl, isoXaZolyl, thiaZolyl, amount of a compound of formula (I) or a pharmaceutically isothiaZolyl, pyraZolyl, imidaZolyl, benZimidaZolyl, acceptable salt form thereof. oXadiaZole, thiadiaZole, triaZole, 1,2,3-oXadiaZole, In a third embodiment, the present invention provides a 1,2,4-oXadiaZole, 1,2,5-oXadiaZole, 1,3,4 novel method for treating or preventing a thromboembolic oXadiaZole, 1,2,3-thiadiaZole, 1,2,4-thiadiaZole, 1,2, 35 disorder, comprising: administering to a patient in need 5-thiadiaZole, 1,3,4-thiadiaZole, 1,2,3-triaZole, 1,2,4 thereof a therapeutically effective amount of a compound of triaZole, 1,2,5-triaZole, and 1,3,4-triaZole; formula (I) or a pharmaceutically acceptable salt form thereof. R2, at each occurrence, is selected from H, CF3, CL6 alkyl, benZyl, C5_6 carbocyclic residue substituted With DEFINITIONS 0—2 R417, and 5—6 membered heterocyclic system con taining from 1—4 heteroatoms selected from the group The compounds herein described may have asymmetric consisting of N, O, and S substituted With 0—2 R4b; centers. Compounds of the present invention containing an R2“, at each occurrence, is selected from H, CF3, CL6 asymmetrically substituted atom may be isolated in optically alkyl, benZyl, phenethyl, C5_6 carbocyclic residue sub active or racemic forms. It is Well knoWn in the art hoW to 45 stituted With 0—2 R417, and 5—6 membered heterocyclic prepare optically active forms, such as by resolution of system containing from 1—4 heteroatoms selected from racemic forms or by synthesis from optically active starting thebgroup consisting of N, O, and S substituted With 0—2 materials. Many geometric isomers of ole?ns, C=N double bonds, and the like can also be present in the compounds R217, at each occurrence, is selected from CF3, CL4 alkoXy, described herein, and all such stable isomers are contem C1_6 alkyl, benZyl, C5_6 carbocyclic residue substituted plated in the present invention. Cis and trans geometric With 0—2 R417, and 5—6 membered heterocyclic system isomers of the compounds of the present invention are containing from 1—4 heteroatoms selected from the described and may be isolated as a mixture of isomers or as grclup consisting of N, O, and S substituted With 0—2 separated isomeric forms. All chiral, diastereomeric, race R4 ; mic forms and all geometric isomeric forms of a structure 55 R26, at each occurrence, is selected from CF3, OH, C1_4 are intended, unless the speci?c stereochemistry or isomeric alkoXy, C1_6 alkyl, benZyl, C5_6 carbocyclic residue form is speci?cally indicated. substituted With 0—2 R417, and 5—6 membered hetero The term “substituted,” as used herein, means that any cyclic system containing from 1—4 heteroatoms one or more hydrogens on the designated atom is replaced selected from the group consisting of N, O, and S With a selection from the indicated group, provided that the substituted With 0—2 R4b; designated atom’s normal valency is not exceeded, and that alternatively, R2 and R2“, together With the atom to Which the substitution results in a stable compound. When a they are attached, combine to form a ring selected from substitent is keto (i.e., :0), then 2 hydrogens on the atom imidaZolyl, morpholino, piperaZinyl, pyridyl, and are replaced. Keto substituents are not present on aromatic pyrrolidinyl, substituted With 0—2 R4b; moieties. 65 R4, at each occurrence, is selected from H, :0, OR2, The present invention is intended to include all isotopes of CHZORZ, F, Cl, C1_4 alkyl, NR2R2“, CH2NR2R2”, C(O) atoms occurring in the present compounds. Isotopes include 6,060,491 11 12 those atoms having the same atomic number but different monocyclic or bicyclic or 7- to 10-membered bicyclic mass numbers. By Way of general example and Without heterocyclic ring Which is saturated partially unsaturated or limitation, isotopes of hydrogen include tritium and deute unsaturated (aromatic), and Which consists of carbon atoms rium. Isotopes of carbon include C-13 and C-14. and from 1 to 4 heteroatoms independently selected from the group consisting of N, O and S and including any bicyclic When any variable (e.g., R6) occurs more than one time group in Which any of the above-de?ned heterocyclic rings in any constituent or formula for a compound, its de?nition is fused to a benZene ring. The nitrogen and sulfur heteroa at each occurrence is independent of its de?nition at every toms may optionally be oxidiZed. The heterocyclic ring may other occurrence. Thus, for example, if a group is shoWn to be attached to its pendant group at any heteroatom or carbon be substituted With 0—2 R6, then said group may optionally atom Which results in a stable structure. The heterocyclic be substituted With up to tWo R6 groups and R6 at each rings described herein may be substituted on carbon or on a occurrence is selected independently from the de?nition of nitrogen atom if the resulting compound is stable. If spe R6. Also, combinations of substituents and/or variables are ci?cally noted, a nitrogen in the heterocycle may optionally permissible only if such combinations result in stable com be quaterniZed. It is preferred that When the total number of pounds. S and O atoms in the heterocycle exceeds 1, then these When a bond to a substituent is shoWn to cross a bond 15 heteroatoms are not adjacent to one another. It is preferred connecting tWo atoms in a ring, then such substituent may be that the total number of S and O atoms in the heterocycle is bonded to any atom on the ring. When a substituent is listed not more than 1. As used herein, the term “aromatic hetero Without indicating the atom via Which such substituent is cyclic system” is intended to mean a stable 5- to 7-membered monocyclic or bicyclic or 7- to 10-membered bonded to the rest of the compound of a given formula, then bicyclic heterocyclic aromatic ring Which consists of carbon such substituent may be bonded via any atom in such atoms and from 1 to 4 heterotams independently selected substituent. Combinations of substituents and/or variables from the group consisting of N, O and S. It is preferred that are permissible only if such combinations result in stable the total number of S and O atoms in the aromatic hetero compounds. cycle is not more than 1. As used herein, “alkyl” is intended to include both Examples of heterocycles include, but are not limited to, branched and straight-chain saturated aliphatic hydrocarbon 25 acridinyl, aZocinyl, benZimidaZolyl, benZofuranyl, groups having the speci?ed number of carbon atoms. benZothiofuranyl, benZothiophenyl, benZoxaZolyl, Examples of alkyl include, but are not limited to, methyl, benZthiaZolyl, benZtriaZolyl, benZtetraZolyl, benZisoxaZolyl, ethyl, n-propyl, i-propyl, n-butyl, s-butyl, t-butyl, n-pentyl, benZisothiaZolyl, benZimidaZolinyl, carbaZolyl, 4aH and s-pentyl. “Haloalkyl” is intended to include both carbaZolyl, carbolinyl, chromanyl, chromenyl, cinnolinyl, branched and straight-chain saturated aliphatic hydrocarbon decahydroquinolinyl, 2H,6H-1,5,2-dithiaZinyl, dihydrofuro groups having the speci?ed number of carbon atoms, sub [2,3-b]tetrahydrofuran, furanyl, furaZanyl, imidaZolidinyl, imidaZolinyl, imidaZolyl, 1H-indaZolyl, indolenyl, stituted With 1 or more halogen (for example —CVFW Where indolinyl, indoliZinyl, indolyl, 3H-indolyl, isobenZofuranyl, v=1 to 3 and W=1 to (2v+1)). Examples of haloalkyl include, isochromanyl, isoindaZolyl, isoindolinyl, isoindolyl, but are not limited to, tri?uoromethyl, trichloromethyl, penta?uoroethyl, and pentachloroethyl. “Alkoxy” represents 35 isoquinolinyl, isothiaZolyl, isoxaZolyl, morpholinyl, naphthyridinyl, octahydroisoquinolinyl, oxadiaZolyl, 1,2,3 an alkyl group as de?ned above With the indicated number oxadiaZolyl, 1,2,4-oxadiaZolyl, 1,2,5-oxadiaZolyl, 1,3,4 of carbon atoms attached through an oxygen bridge. oxadiaZolyl, oxaZolidinyl, oxaZolyl, oxaZolidinyl, Examples of alkoxy include, but are not limited to, methoxy, pyrimidinyl, phenanthridinyl, phenanthrolinyl, phenaZinyl, ethoxy, n-propoxy, i-propoxy, n-butoxy, s-butoxy, t-butoxy, phenothiaZinyl, phenoxathiinyl, phenoxaZinyl, phthalaZinyl, n-pentoxy, and s-pentoxy. “Cycloalkyl” is intended to piperaZinyl, piperidinyl, pteridinyl, purinyl, pyranyl, include saturated ring groups, such as cyclopropyl, pyraZinyl, pyraZolidinyl, pyraZolinyl, pyraZolyl, cyclobutyl, or cyclopentyl. Alkenyl” is intended to include pyridaZinyl, pyridooxaZole, pyridoimidaZole, hydrocarbon chains of either a straight or branched con?gu pyridothiaZole, pyridinyl, pyridyl, pyrimidinyl, pyrrolidinyl, ration and one or more unsaturated carbon-carbon bonds pyrrolinyl, 2H-pyrrolyl, pyrrolyl, quinaZolinyl, quinolinyl, 45 Which may occur in any stable point along the chain, such 4H-quinoliZinyl, quinoxalinyl, quinuclidinyl, as ethenyl, propenyl and the like. “Alkynyl” is intended to tetrahydrofuranyl, tetrahydroisoquinolinyl, include hydrocarbon chains of either a straight or branched tetrahydroquinolinyl, 6H-1,2,5-thiadiaZinyl, 1,2,3 con?guration and one or more triple carbon-carbon bonds thiadiaZolyl, 1,2,4-thiadiaZolyl, 1,2,5-thiadiaZolyl, 1,3,4 Which may occur in any stable point along the chain, such thiadiaZolyl, thianthrenyl, thiaZolyl, thienyl, thienothiaZolyl, as ethynyl, propynyl and the like. thienooxaZolyl, thienoimidaZolyl, thiophenyl, triaZinyl, 1,2, “Halo” or “halogen” as used herein refers to ?uoro, 3-triaZolyl, 1,2,4-triaZolyl, 1,2,5-triaZolyl, 1,3,4-triaZolyl, chloro, bromo, and iodo; and “counterion” is used to rep and xanthenyl. Preferred heterocycles include, but are not resent a small, negatively charged species such as chloride, limited to, pyridinyl, furanyl, thienyl, pyrrolyl, pyraZolyl, bromide, hydroxide, acetate, sulfate, and the like. pyrrolidinyl, imidaZolyl, indolyl, benZimidaZolyl, 55 As used herein, “carbocycle” or “carbocyclic residue” is lH-indazolyl, oxaZolidinyl, benZotriaZolyl, benZisoxaZolyl, intended to mean any stable 3- to 7-membered monocyclic oxindolyl, benZoxaZolinyl, or isatinoyl. Also included are or bicyclic or 7- to 13-membered bicyclic or tricyclic, any of fused ring and Spiro compounds containing, for example, Which may be saturated, partially unsaturated, or aromatic. the above heterocycles. Examples of such carbocycles include, but are not limited to, The phrase “pharmaceutically acceptable” is employed cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, herein to refer to those compounds, materials, compositions, cycloheptyl, adamantyl, cyclooctyl, [3.3.0]bicyclooctane, and/or dosage forms Which are, Within the scope of sound [4.3.0]bicyclononane, [4.4.0]bicyclodecane, [2.2.2] medical judgment, suitable for use in contact With the tissues bicyclooctane, ?uorenyl, phenyl, naphthyl, indanyl, of human beings and animals Without excessive toxicity, adamantyl, or tetrahydronaphthyl. 65 irritation, allergic response, or other problem or As used herein, the term “heterocycle” or “heterocyclic complication, commensurate With a reasonable bene?t/risk system” is intended to mean a stable 5- to 7-membered ratio. 6,060,491 13 14 As used herein, “pharmaceutically acceptable salts” refer “Therapeutically effective amount” is intended to include to derivatives of the disclosed compounds Wherein the an amount of a compound of the present invention or an parent compound is modi?ed by making acid or base salts amount of the combination of compounds claimed effective thereof. Examples of pharmaceutically acceptable salts to inhibit HIV infection or treat the symptoms of HIV include, but are not limited to, mineral or organic acid salts infection in a host. The combination of compounds is of basic residues such as amines; alkali or organic salts of preferably a synergistic combination. Synergy, as described acidic residues such as carboxylic acids; and the like. The for example by Chou and Talalay, Adv. EnZyme Regul. pharmaceutically acceptable salts include the conventional 22:27—55 (1984), occurs When the effect (in this case, non-toxic salts or the quaternary ammonium salts of the inhibition of HIV replication) of the compounds When parent compound formed, for example, from non-toxic administered in combination is greater than the additive inorganic or organic acids. For example, such conventional effect of the compounds When administered alone as a single non-toxic salts include those derived from inorganic acids agent. In general, a synergistic effect is most clearly dem such as hydrochloric, hydrobromic, sulfuric, sulfamic, onstrated at suboptimal concentrations of the compounds. phosphoric, nitric and the like; and the salts prepared from organic acids such as acetic, propionic, succinic, glycolic, Synergy can be in terms of loWer cytotoxicity, increased stearic, lactic, malic, tartaric, citric, ascorbic, pamoic, 15 antiviral effect, or some other bene?cial effect of the com maleic, hydroxymaleic, phenylacetic, glutamic, benZoic, bination compared With the individual components. salicylic, sulfanilic, 2-acetoxybenZoic, fumaric, toluenesulfonic, methanesulfonic, ethane disulfonic, oxalic, SYNTHESIS isethionic, and the like. The compounds of the present invention can be prepared The pharmaceutically acceptable salts of the present invention can be synthesiZed from the parent compound in a number of Ways knoWn to one skilled in the art of organic synthesis. The compounds of the present invention Which contains a basic or acidic moiety by conventional chemical methods. Generally, such salts can be prepared by can be synthesiZed using the methods described beloW, reacting the free acid or base forms of these compounds With together With synthetic methods knoWn in the art of syn a stoichiometric amount of the appropriate base or acid in 25 thetic organic chemistry, or variations thereon as appreciated Water or in an organic solvent, or in a mixture of the tWo; by those skilled in the art. Preferred methods include, but are generally, nonaqueous media like ether, ethyl acetate, not limited to, those described beloW. The reactions are ethanol, isopropanol, or acetonitrile are preferred. Lists of performed in a solvent appropriate to the reagents and suitable salts are found in Remington’s Pharmaceutical materials employed and suitable for the transformations Sciences, 17th ed., Mack Publishing Company, Easton, Pa., being effected. It Will sometimes require a judgment to 1985, p. 1418, the disclosure of Which is hereby incorpo modify the order of synthetic steps or to select one particular rated by reference. process scheme over another in order to obtain a desired “Prodrugs” are intended to include any covalently bonded compound of the invention. It Will also be recogniZed that carriers Which release the active parent drug according to another major consideration in the planning of any synthetic formula (I) in vivo When such prodrug is administered to a route in this ?eld is the judicious choice of the protecting 35 mammalian subject. Prodrugs of a compound of formula (I) group used for the protection of the reactive functional are prepared by modifying functional groups present in the groups present in the compounds described in this invention. compound in such a Way that the modi?cations are cleaved, An authoritative account describing the many alternatives to either in routine manipulation or in vivo, to the parent the trained practitioner is Greene and Wuts (Protective compound. Prodrugs include compounds of formula (I) Groups in Organic Chemistry, Wiley and Sons, 1991). All Wherein a hydroxy, amino, or sulfhydryl group is bonded to references cited herein are hereby incorporated in their any group that, When the prodrug or compound of formula entirety herein by reference. Compounds of this invention (I) is administered to a mammalian subject, cleaves to form Where B is either a carbocyclic or heterocyclic residue as a free hydroxyl, free amino, or free sulfhydryl group, de?ned in Formula 1 are coupled to A as shoWn generically respectively. Examples of prodrugs include, but are not and by speci?c example in Schemes 1 and 2, respectively. 45 limited to, acetate, formate and benZoate derivatives of Either or both of A and B may be substituted With 0—2 R4. alcohol and amine functional groups in the compounds of W is de?ned as a suitable protected nitrogen, such as NO2 formula (I), and the like. Preferred prodrugs are amidine or NHBOC; a protected sulfur, such as S-tBu or SMOM; or prodrugs Wherein D is C(=NR7)NH2, and R7 is selected a methyl ester. Halogen-metal exchange of the bromine in from OH, C1_4 alkoxy, C6_1O aryloxy, C1_4 alkoxycarbonyl, bromo-B With n-butyl lithium, quenching With triisopropyl C6_1O aryloxycarbonyl, C6_1O arylmethylcarbonyl, C1_4 alky borate and acidic hydrolysis gives the required boronic acid, lcarbonyloxy C1_4 alkoxycarbonyl, and C6_1O arylcarbony B—B(OH)2. The W—A—Br subunit may be already linked loxy C1_4 alkoxycarbonyl. More preferred prodrugs are to ring M before the SuZuki coupling reaction. Deprotection Where R7 is OH, methoxy, ethoxy, benZyloxycarbonyl, provides the complete subunit. methoxycarbonyl, and methylcarbonyloxymethoxycar 55 bonyl. Scheme 1 “Stable compound” and “stable structure” are meant to B—Br indicate a compound that is suf?ciently robust to survive 1) n-BuLi isolation to a useful degree of purity from a reaction mixture, 2) (iPrO)3B and formulation into an ef?cacious therapeutic agent. 3) HCI “Substituted” is intended to indicate that one or more hydrogens on the atom indicated in the expression using “substituted” is replaced With a selection from the indicated I group(s), provided that the indicated atom’s normal valency A Pdm) W—A—B is not exceeded, and that the substitution results in a stable 65 l compound. When a substituent is keto (i.e., :0) group, then Br 2 hydrogens on the atom are replaced. 6,060,491 15 16 -continued Scheme 1 TABLE A-continued Preparation of Amide Ester, Urea, Sulfonamide and Sulfamide Linkages Between A and B. then the reactive to give the following If A contains: substituent of Y is: product A—X—Y: a secondary NH ClC(O)—Y A—C(O)—Y as part of a Scheme 2 describes a typical example of how the A-B ring or chain A—OH as a ClC(O)—Y A—O—C(O)—Y subunit is prepared for attachment to ring M. substituent 4-Bromoaniline is protected as Boc-derivative and the A—NHR2 as a C1C(O)—CR2R2“— A—NR2—C(O)—CR2R2“—Y coupled to 2-(t-butylamino)sulfonylphenylboronic acid substituent —Y under Suzuki conditions. 2-(t-Butylamino) 15 a secondary NH ClC(O)—CR2R2“— A—C(O)—CR2R2“—Y as part of a —Y sulfonylphenylboronic acid is prepared by the method ring or chain described by Rivero (Bioorg. Med. Chem. Lett. 1994, 189). A—OH as a ClC(O)—CR2R2“— A—O—C(O)—CR2R2“—Y Deprotection with TFA can provide the aminobiphenyl com substituent —Y A—NHR2 as a ClC(O)—CNR2—Y A—NR2—C(O)—CNR2—Y pound. The aminobiphenyl is then coupled to the core ring substituent structures as described below. a secondary NH C1C(O)—CNR2—Y A—C(O)—CNR2—Y as part of a ring or chain Scheme 2 A—OH as a C1C(O)—CNR2—Y A—O—C(O)—CNR2—Y B(OH)2 substituent 25 A—NHR2 as a ClSO2—Y A—NR2—SO2—Y NH; NHBOC SOZNH-t-Bu substituent a secondary NH ClSO2—Y A—SO2—Y NaH as part of a (BOC)2O ring or chain —> Pd(0) A—NHR2 as a ClSO2—CR2R2“— A—NR2—SO2—CR2R2“—Y substituent —Y a secondary NH C1SO2—CR2R2“— A—SO2—CR2R2“—Y Br Br as part of a —Y NHBOC NHZ ring or chain A—NHR2 as a ClSO2—NR2—Y A—NR2—SO2—NR2—Y substituent a secondary NH ClSO2—NR2—Y A—SO2—NR2—Y as part of a ring or chain TFA —> A—C(O)Cl HO—Y as a A—C(O)—O—Y substituent SOZNH-t-Bu SOZNH-t-Bu A—C(O)Cl NHR2—Y as a A—C(O)—NR2—Y substituent A—C(O)Cl a secondary NH as A—C(O)—Y part of a ring or chain A—CR2R2“C(O)C1 HO—Y as a A—CR2R2“C(O)—O—Y When B is de?ned as X—Y, the following description substituent applies. Groups A and B are available either through com A—CR2R2“C(O)Cl NHR2—Y as a A—CR2R2“C(O)—NR2—Y substituent mercial sources, known in the literature or readily synthe A—CR2R2“C(O)Cl a secondary NH as A—CR2R2“C(O)—Y siZed by the adaptation of standard procedures known to part of a ring or practitioners skilled in the art of organic synthesis. the chain required reactive functional groups appended to analogs of A—SO2C1 NHR2—Y as a A—SO2—NR2—Y A and B are also available either through commercial substituent sources, known in the literature or readily synthesiZed by the A—SOZCl a secondary NH as A—SO2—Y part of a ring or adaptation of standard procedures known to practitioners chain skilled in the art of synthesis. In the tables that follow the 55 A—CR2R2“SO2C1 NHR2—Y as a A—CR2R2“SO2—NR2—Y chemistry required to effect the coupling of A to B is substituent outlined. A—CRZRZQSOZCl a secondary NH as A—CR2R2“SO2—Y part of a ring or TABLE A chain Preparation of Amide Ester, Urea, Sulfonamide and Sulfamide Linkages Between A and B. then the reactive to give the following If A contains: substituent of Y is: product A—X—Y: The chemistry of Table A can be carried out in aprotic A—NHR2 as a C1C(O)—Y A—NR2—C(O)—Y 65 solvents such as a chlorocarbon, pyridine, benZene or substituent toluene, at temperatures ranging from —20° C. to the re?ux point of the solvent and with or without a trialkylamine base. 6,060,491 17 18 Lett. 1992, 235). The sulfone can be prepared according to TABLE B the method of Satoh (Chem. Lett. 1992, 381) using m-chloroperbenzoic acid. Preparation of Ketone Linkages between A and B. Scheme 3 describes the synthesis of compounds Wherein M is a benzene ring and Q is a protected precursor of group then the reactive to give the following D of Formula I and V is a nitro, protected sulfonamide or ester group and precursor of group Z of Formula I. The V group is placed on an appropriately substituted phenol either via nitration as shoWn by Poirier et al. (Tetrahedron 1989, 45(5), 1415), sulfonylation as shoWn by Kuznetsov (Akad. 10 Nauk SSSR Ser. Khim 1990, 8, 1888) or carboxylation by Sartori et al. (Synthesis 1988, 10, 763). Bromination With The coupling chemistry of table B can be carried out by triphenylphosphine and bromine (J. Am. Chem. Soc. 1964, a variety of methods. The Grignard reagent required for Y is 86, 964) gives the desired bromide. Suzuki coupling With the prepared from a halogen analog of Y in dry ether, appropriate boronic acid provides the desired substituted dimethoxyethane or tetrahydrofuran at 0° C. to the re?ux 15 pyridine. point of the solvent. This Grignard reagent can reacted directly under very controlled conditions, that is loW tem Scheme 3 perature (—20° C. or loWer) and With a large excess of acid chloride or With catalytic or stoichiometric copper bromide'dimethyl sul?de complex in dimethyl sul?de as a 20 solvent or With a variant thereof. Other methods available include transforming the Grignard reagent to the cadmium reagent and coupling according to the procedure of Carson and Prout (Org. Syn. Col. Vol. 3 (1955) 601) or a coupling mediated by Fe(acac)3 according to Fiandanese et al. (Tern 25 Lett. 1984, 4805), or a coupling mediated by manganese (II) catalysis (Cahiez and Laboue, Terr. Lett. 1992, 33(31), 4437). TABLE C 30 Schemes 4, 5, 6, and 7 describe the synthesis of com pounds Wherein M is pyridine and Q is a protected precursor Preparation of Ether and Thioether linkages betWeen A and B. of group D of Formula I. Each scheme represents a different substitution pattern for the pyridine ring. In Scheme 4, a then the reactive to give the folloWing suitably protected aldehyde is subjected to base-catalyzed 35 condensation With an activated ester to give after deprotec tion the desired aldehyde. Re?uxing With ammonium chlo ride as shoWn by DornoW and Ische (Chem. Ber. 1956, 89, 876) provides the pyridinol Which is brominated With POBr3 (Tjeenk et al. Rec. Trav. Chim. 1948, 67, 380) to give the 40 desired 2-bromopyridine. Suzuki coupling With the appro priate boronic acid provides the desired substituted pyridine. The ether and thioether linkages of Table C can be Scheme 4 prepared by reacting the tWo components in a polar aprotic coR1b solvent such as acetone, dimethylformamide or dimethyl 45 sulfoxide in the presence of a base such as potassium o carbonate, sodium hydride or potassium t-butoxide at a </ CH0 1) NaOEt temperature ranging from ambient to the re?ux point of the solvent used. O EtOZC v —> or 2) H30+ TABLE D Preparation of —SO— and —SO2— linkages from O thioether of Table C. then it is oxidized then it is oxidized 55 With Wet With m If the starting Alumina/Oxone to chloroperbenzoic acid material is: give: to give: A—S—Y A—S(O)—Y A—SO2—Y 60 The thioethers of Table C serve as a convenient starting material for the preparation of the sulfoxide and sulfone analogs of Table D. A combination of Wet alumina and 65 Oxone can provide a reliable reagents for the oxidation of Treatment of an appropriately substituted the thioether to the sulfoxide as shoWn by Greenhalgh (Syn. 5 -ethoxyoxazole With an alkene as shoWn by Kondrat’eva et
Description: