15.1 Amines Amine PDF
Preview 15.1 Amines Amine
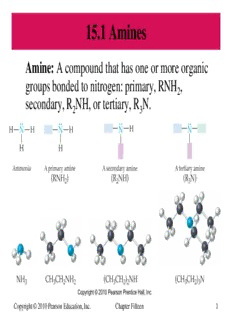
15.1 Amines Amine: A compound that has one or more organic groups bonded to nitrogen: primary, RNH , 2 secondary, R NH, or tertiary, R N. 2 3 Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 1 Primary alkyl amines are named by identifying the alkyl group attached to nitrogen and adding the suffix -amine to the alkyl group name. Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 2 Simple, secondary and tertiary amines (those possessing two or three identical groups on the nitrogen, respectively) are named by adding the appropriate prefix, di- or tri-, to the alkyl group name along with the suffix -amine. Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 3 ► When R groups in 2 or 3 amines are different, the compounds are named as N-substituted derivatives of a 1 amine. The parent compound is chosen as the 1 amine that contains the largest of the R groups. ► All other groups are considered to be N-substituents. The following compounds are named as propylamines because the propyl group in each is the largest alkyl group: Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 4 ► The simplest aromatic amine is known by the common name aniline. ► The –NH functional group is an amino group, and 2 when this group is a substituent, amino- is used as a prefix. Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 5 15.2 Properties of Amines The lone electron pair on the N in amines causes amines to be weak Brønsted–Lowry bases or Lewis bases, electron pair donors, by forming a bond with an H+ ion from an acid or water. Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 6 ► Protein in flesh contains amine groups. Volatile amines produced during decay are responsible for the odor of rotten fish or decaying meat. Cadaver-sniffing dogs are used to detect the strong odor of the amines produced from decaying flesh. ► Many amines cause physiological responses. Simpler amines are irritating and toxic. Some complex amines from plants can be very poisonous. Many useful . drugs are amines Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 7 ► Because of hydrogen bonding, 1 and 2 amines have higher boiling points than alkanes of similar size. Amines are lower boiling than alcohols of similar size. ► All amines can hydrogen-bond to water molecules through the lone electron pair on their nitrogen atoms. Amines with up to about 6 C’s are soluble in water. Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 8 3 amine molecules have no H atoms attached to N and therefore cannot hydrogen-bond with each other. As a result they are much lower boiling than alcohols or 1 or 2 amines of similar molecular weight. Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 9 15.3 Heterocyclic Nitrogen Compounds ► Heterocycle: A ring that contains nitrogen or some other atom in addition to carbon. ► Heterocycles are common in many natural compounds found in plants and animals. Copyright © 2010 Pearson Education, Inc. Chapter Fifteen 10
Description: