1, trans-trans, anti-anti 2, trans-cis, anti-anti 3, cis-cis, anti-anti 4, trans-trans, syn-anti 5, trans-cis PDF
Preview 1, trans-trans, anti-anti 2, trans-cis, anti-anti 3, cis-cis, anti-anti 4, trans-trans, syn-anti 5, trans-cis
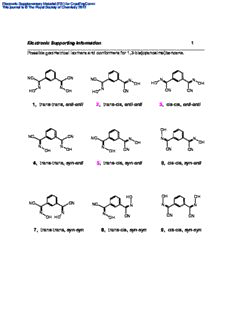
Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 Electronic Supporting Information 1 ______________________________________________________________________ Possible geometrical isomers and conformers for 1,3-bis(cyanoxime)benzene. NC CN NC N N N OH HO OH N N N HO OH HO CN CN CN 1, trans-trans, anti-anti 2, trans-cis, anti-anti 3, cis-cis, anti-anti OH NC CN NC N N N OH OH N N N OH OH OH CN CN CN 4, trans-trans, syn-anti 5, trans-cis, syn-anti 6, cis-cis, syn-anti HO OH OH NC CN NC N N N N N N OH HO OH CN CN CN 7, trans-trans, syn-syn 8, trans-cis, syn-syn 9, cis-cis, syn-syn Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 Electronic Supporting Information 2 ______________________________________________________________________ Possible geometrical isomers and conformers for 1,4-bis(cyanoxime)benzene. OH N CN CN OH N NC NC N N HO HO I, trans, anti-anti II. cis, anti-anti HO N CN CN N NC NC OH N N HO HO III, trans, syn-anti IV, cis, syn-anti HO N CN CN N NC NC OH N N OH OH V, trans, syn-anti VI, cis, syn-anti Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 Electronic Supporting Information 3 ______________________________________________________________________ Crystal data and structure refinement for the 1,3-BCO-monooxime. _____________________________________________________________________________________ Empirical formula C H N O 10 7 3 Formula weight 185.19 Temperature 296(2) K Wavelength 0.71073 Å Crystal system Triclinic Space group P -1, #2 Unit cell dimensions a = 5.676(4) Å = 102.487(7)°. b = 9.573(7) Å = 101.186(7)°. c = 9.600(7) Å = 106.568(7)°. 3 Volume, Z 469.6(6) Å , 2 3 Density (calculated) 1.310 Mg/m -1 Absorption coefficient 0.090 mm F(000) 192 Index ranges -6<=h<=6, -11<=k<=11, -11<=l<=11 Reflections collected 3673 Independent reflections 1764 [R(int) = 0.0462] Completeness to theta = 25.67° 98.7 % Max. and min. transmission 0.7454 and 0.4930 Refinement method Full-matrix least-squares on F2 Data / restraints / parameters 1764 / 0 / 139 Goodness-of-fit on F2 0.997 Final R indices [I>2sigma(I)] R1 = 0.0696, wR2 = 0.1575 R indices (all data) R1 = 0.1390, wR2 = 0.1938 Extinction coefficient 0.029(13) -3 Largest diff. peak and hole 0.451 and -0.165 e.Å _____________________________________________________________________________________ Hydrogen bonds for 1,3-BCO-monooxime [Å and °]. _____________________________________________________________________________________ D-H...A d(D-H) d(H...A) d(D...A) <(DHA) _____________________________________________________________________________________ O(1)-H(1O)...N(3)#1 1.09(6) 1.67(6) 2.749(4) 172(4) _____________________________________________________________________________________ Symmetry transformations used to generate equivalent atoms: #1 -x+1,-y+1,-z Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 Electronic Supporting Information 4 ______________________________________________________________________ Molecular structure and numbering scheme for the 1,3-BCO-monooxime. An ORTEP drawing at 50% thermal ellipsoids probability level. Selected bonds lengths (Å) are indicated in yellow. Actual TLC (MeOH/CHCl3) of all compounds involved into the synthesis of 1,3-BCO. Rf = 0.69 NC CN NC Rf = 0.39 N CN HO Rf = 0.16 NC CN N N HO OH Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 s n o b r a c N 52 C p s , p e s H h O m t n pp K i N 1 7 93 n. C 22. 2 o N t at 6 oluti glet a o-d n s + sin s i a m s N s d er C e i m r n e e i so p h m i u xi cal gro monoo ometri N he CH‐2 CO- o ge NC HO T B w - t 3 f 1, o e e c h n t e of es e r pl p m e h a t s d t e o c p n s e C d L vi T e on e 0 i n 1 at o of m e, d or ur a f p e n f st I o n g m i n s orti ctru gnal pp pe si u s 5 S R 1 c M e ni N Th ro H} n. ct1 o e C{ gi El3 e 1 r Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 6 0 2 2 K. 6 2 3 2 .7 9 2 2 2 4 t a 2 6 d - o s m d N n 1 C i ) rile 2 m nit 5 3 o p et 6 p s-ac 6 4 1 19.4 20 t, g bi 1 1 hif n ti s star C al ( N c e d i ni m a y e c 5 di 2 h yl- 1 c n e C h p 4 3 yl 8 1 nz 5 27. n be 1 07 io di 48. at 3- 2 m , 1 0 1 nfor ure .01 13 p 6 0 I 3 g f o 1 n i a ort ctr 0 p e 5 up sp 3 32. S R 1 c M 5 ni N 3 o } 1 r H ct1 { e C El3 1 Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 s w o r r a 7 __ ed __ R _ . ____ etails _ d ___ B – _ ____ view, ___ mic _ a _ r _ o _ n _ a _ p _ – __ A __ K: _ _ 3 _ 9 _ 2 _ t _ a H __ d 6 O N __ o- N _ ms C _ _ d _ n __ O i yn- _ s _ C _ B . _____ e 1,3- somer OH N CN _ h i _ t al ___ e of tric + __ mpl me _ o __ sa ge N H _ t C O n __ po nd o_ s o N i_ C c t e a_ L s m_ T nfor____ one of the anti- g I__ e, e tin___ pur enc N ppor___ a of pres NC HO ic Su_____ spectr minor on__ R e ctr__ NM cat Ele___ 1H indi Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 d e 8 c n e H d O N vi e N C s e n of li n- t sy e s N ble C H u o O N D . K 3 + 9 2 t a N H 6 C O d - so N m d n - i ti O n a C B 3- N , 1 e C O h N H t f o e pl mn. ao t suti sposol C n n Ls i tio e Tmer a n m oo s nfor ure,cal i I pri g of et n m orti ctraeo p eg p po u sw S Rt c Mof ni Ne ro H} nc ct1{se e Ce El13 pr Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 Electronic Supporting Information 9 ______________________________________________________________________ UV-visible spectra of 1,3-BCO (top) and 1,4-BCO (nbottom) in different solvents upon addition of KOH with the formation of dianion. Shown n→* and n→* (only for 1,3- BCO) transitions in visible region of spectrum. 0,3 CN PrOH OK MeOH N CHCN 3 EtOH OK DMSO NC N 0,2 DMF e c n a b or n * s 0,1 b n * A 0,0 400 500 600 700 800 Wavelength (nm) 1,00 MeOH EtOH PrOH DMF 0,75 DMSO e c O- K+ n 0,50 N a b CN r o bs n * NC A 0,25 N +K -O 0,00 400 500 600 700 Wavelength (nm) Electronic Supplementary Material (ESI) for CrystEngComm This journal is © The Royal Society of Chemistry 2012 Electronic Supporting Information 10 ______________________________________________________________________ Tables of H-bonding for studied bis-cyanoximes. 1,4-BCO · H O [Å and °]. 2 _____________________________________________________________________ D-H...A d(D-H) d(H...A) d(D...A) <(DHA) ____________________________________________________________________________ O(1)-H(1)...O(3S)#1 0.84 1.93 2.748(3) 165.5 O(3S)-H(1S)...N(4)#2 0.67(4) 2.20(4) 2.851(4) 167(5) ____________________________________________________________________________ Symmetry transformations used to generate equivalent atoms: #1 -x+3/4,y+1/4,z+3/4; #2 x,y,z+1 1,3-BCO · DMF [Å and °]. ____________________________________________________________________________ D-H...A d(D-H) d(H...A) d(D...A) <(DHA) ____________________________________________________________________________ O(1)-H(1)...O(1S) 0.84 1.83 2.664(2) 169.7 O(1)-H(1)...O(1S)#2 0.84 1.83 2.664(2) 169.7 ____________________________________________________________________________ Symmetry transformations used to generate equivalent atoms: #1 x,y,-z+3/2 #2 x,y,-z+1/2 1,4-BCO [Å and °]. ____________________________________________________________________________ D-H...A d(D-H) d(H...A) d(D...A) <(DHA) ____________________________________________________________________________ O(1)-H(1)...N(2)#2 0.84 1.99 2.814(2) 165.6 ____________________________________________________________________________ Symmetry transformations used to generate equivalent atoms: #1 -x+1,-y,-z+1 #2 -x-1/2,y+1/2,z 1,4-BCO · DMSO [Å and °]. ____________________________________________________________________________ D-H...A d(D-H) d(H...A) d(D...A) <(DHA) ____________________________________________________________________________ O(1)-H(1)...O(1S)#2 0.92(2) 1.68(2) 2.6044(14) 176(2) ____________________________________________________________________________ Symmetry transformations used to generate equivalent atoms: #1 -x+1,-y+2,-z+2 #2 x+1,y,z
Description: