Why Liquid Oxygen Is Blue PDF
Preview Why Liquid Oxygen Is Blue
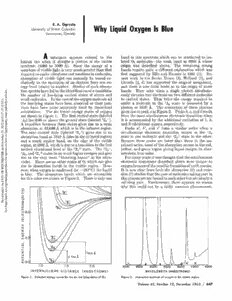
E. A. Ogryzlo University of British Columbia Why Liquid Oxygen Is Blue Vancouver, Canada M substance appears colored to the band in this spectrum which can be attributed to iso- human eye when it absorbs a portion of the visible lated 02 molecules—the weak band at 6990 A whose spectrum (4000 to 7000 A). Since the energy of a origin was described above. The remaining strong quantum of visible light isvery much greater than that bands require quite a different explanation which was requiredto excitevibrations and rotations inmolecules, first suggested by F41is and Ivnesser in 1933 (1). Re- absorption of visible light can normally be traced ex- cent work in the Soviet Union {%), Holland (5), and clusively to the excitation of an electron from one en- Canada (4-, 5) has supported the original assignment, ergy level (state) to another. Studies of such absorp- and there is now little doubt as to the origin of these es. tion spectrahaveledto theidentificationofa considera- bands. They arise when a single photon simultane- 7 (UTC).ublished articl bsttihmoleenasllnloumhwmao-vlblyeeeicnrubgleeoessfn.talotqewIusn-itlhytehainevageccacbseueexrecaonittfeeotldhybessfeitoxarxevtyeedgsdebnooyrfmatthohtoeleemoicrrsuelpetaoicnasadil-ll otepoxuhcsoeilttyxoecneiatleeadvmtaots6elt3eas4cte0tuwsle.oA.teoTlehTctuhhtsreeontwa:sAbigcosenosrtttpawhtteoieonedisniofefferpgrotehysensresteesmqseupodihlreeocbdtuoylnetosas 7:3e p calculations. The six lowest energy states of oxygen givesriseto peaka inFigure2. Peaksb, c, anddresult 2:1har are shown in Figure 1. The first excited state (labeled from the same simultaneous electronic transition when mber 25, 2022 at 0w to legitimately s AaTa1Abbhasset)rooalrrnineppessttxiiioott0inno.e9nba8xatcbeintve1ed2tdwa,a6ebts9eot07anv6teAe1tht9hwe(lseAahebigc(esrahlotelasudiostnedsii1nnSsgfttftih+hva)eeetesgiinni(rvflifsareraeabsrreeetrloeiddsdearree3tgwgo2iieo„o”aann)nk).. iasstitnmaPdistuee3laataikcnvnscibeooroamnaeu',ptsiaombnn'e,oiaelleledaccnqtubrduolyeancntiahc'tanefdotrraeratmdhsndpeseiiatticlio2otisnfnvfia+emllyoislec.atcaxrutcresistaeinrtiiteootsnhewtohfheoet1hn,1eA2ra.,f Noveon ho arengdiona,matu6c9h90wAea,kwehricbhanisdd,uoentothaetreadngseitioofntthoethveisfiibrslet Bpreicmaeudsesethrieesse, mpoesatksofatrheelaobwseorrpthtiaonn otchcousrse iinn tthheeruend-, ER on ptions e3Axc„,itaenddv3ib2ura+tsiotantaesl lleievealt omfutchheh1i2gfhf+ersetanteer.giesThaend1giv—e, yaecltleorwis,ticanbdlugereceonlorre.gion giving liquid oxygen its char- HESTs for o vriisoeletto. thTehevreeryarewenaoko“tHheerrzsbtaetregsboafn0d2sw”hinichthceanulgtrivae- eleFcotrromniacnytraynesairtsiointswadsesthcroibuegdhtatbhoavtethweeresimuunltiaqnueeoutos V OF ROCngguideline reisivseebrl,utowe.haebnTshooerxpytgaioebnnsoibsrapcntoidonsndeibnnasnethddes(awvtihs~iibc1hle83arr°eeCgi)orentsh.peolnHiqsoiubwilde- oIstixoynisge(n7no)wbsetcucadleuieasser fotrhfoatmhtetbhpoeotsphsaibtihrleeoffoambrmsoolaertcpioutinloensoft(aa6nk)i0na.gnisdppaeecrmtieisisn-. NIari for this color are shown in Figure 2. There is only one thisprocessare not boundto eachotherbut are simplya nloaded via Uubs.acs.org/sh wcohlylidtihnigspcaoiur.ld nFoutrthbeermaofraeir,lythecormemaopnpeaprhsennoomreeansoonn. wp Dos:// p htt e e S WAVELENGTH (ANGSTROMS) Figure 1. Potential energy curves for thesix low lying states ofO2. Figure2. Absorption spectrum of oxygen in the visible region. Volume 42, Number 12, December 1965 / 647 The reason why it is seldom observed is that twice the systems, undoubtedly, additional simultaneous elec- energy of a given electronic transition is almost always tronic transitions will be discovered. in a region where another strong transition dominates the spectrum. However, there are at least two other Literature Cited examples of simultaneous electronic transitions in the literature. In 1961 (8) a simultaneouselectronictransi- ((12)) EDlilaisn,oJv.-KWl.o,kaondv,KVn.eIs.,seOrp,t.Hi.SOp.,eZct.roPshkyospiikya,,866,,548537((11993539)).. tion was reported for two Pr3+ions in a solid crystal of (3) Fahrenfort, J., Thesis, University ofAmsterdam, 1955. PrCl;;. The striking thing about this observation is (4) Landau, A., Allin, E. J., and Welsh, H. L., Spectrochim. that the two Pr3+ ions that are excited by a single pho- Acta, 18, 1 (1962). ton are separated from one another by chloride ions. (5) Bader, L. W., and Ogryzlo, E. A., Discussions Faraday Soc.,37,46(1964). The other system for which a simultaneous electronic (6) Salow, H., and Steiner, W., Z. Physik, 99, 137 (1936). transitionhasbeenreportedisa solutionofnaphthalene (7) Arnold, S. J., Browne, It. J., and Ogryzlo, E. A., J. and oxygen in chloroform (9). In this system a 3500 A PhotochemistryandPhotobiology, December, 1965. photon was found to simultaneously excite 02 to the (8) Vansanyi, F., and Dieke, G. H., Phys. Rev. Letters, 7, 442 (1961). 1Ag state and naphthalene to the lowest triplet state (9) Dijkgraaf, C., Sitters, R., and Hoijtink, G. J., Mol. (3£>2„). As more careful studies are made of other Phys.,5,643(1962). 648 / Journal of Chemical Education
